This website uses cookies to ensure you get the best experience on our website.
Read more
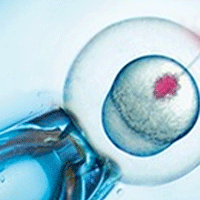
Microinjection Tool Box
January 29, 2021


The system depicted includes components often favored by researchers:
- MICRO-ePUMP Pneumatic PicoPump with built in MICRO-ePORE™ Cell Penetrator to facilitate microinjections
- SU-P1000 Micropipette Puller
- M4C Stand
- M3301R Micromanipulator
- PZMTIII Microscope with Optional Lighted Base with Articulating Mirror and optional PRO-300 HDS Camera and View Screen
- E2XX Micropipette Storage Jar
- Z-MOLDS Microinjection and Transplantation Molds
- 14003-G Vannas spring scissors
- Glass Capillaries
- 77020 Glass Tweezers
- FluoroDish Optical Grade Glass Bottom Culture Dishes
Whatever your needs, WPI offers a range of equipment to fill your requirements.
Empowering Scientists with Reliable Instruments
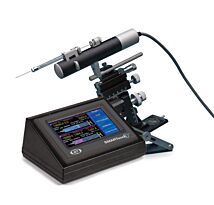
Serving scientists for over 50 years, WPI offers a variety of instruments for microinjection including pumps, pipetters, microscopes and more. One of our most popular pumps for microinjection is the PV850 Pneumatic PicoPump.
The PV850, PV830, uPump and MICRO-ePUMP were designed to simplify intracellular injection. You get repeatable microinjection in volumes ranging from picoliters to nanoliters. The volume injected is controlled by the inside diameter of the glass tip, the pressure and the time.
- The PV850 offers eject and hold pressure. The hold pressure prevents backfilling of the pipette by capillary action, ensuring repeatable injections.
- In addition, the PV830 also has vacuum pressure which allows you to securely hold a cell with one pipette while you inject it with another.
- The uPUMP and MICRO-ePUMP have a built in pressure source, eliminating the need for an external pressure supply. The MICRO-ePUMP with Integrated Cell Penetrator and Internal Pressure Source is perfect for intracellular injection. The Cell Penetrator delivers a highly localized voltage signal to a targeted injection site to facilitate penetration with minimal trauma.
WPI has a customizable Microinjection System with everything you need to get started. The basic system is shown here. Below you will find many options and accessories to customize your system.
Options
You may customize your system using the following options:
MICROSCOPES
- PZMIII Precision Stereo Zoom Microscope on Track Stand
- PZMIV Precision Stereo Zoom on Track Stand
- PZMIII-MI Stereo Microscope with LED Illuminated Base and Articulating Mirror
- 504928 LED Lighted Microscope Stand, 12.5"
- 504929 LED Lighted Microsccope Stand, 10.5"
- 504596 Halogen Lighted Microscope Stand
INJECTOR
- PV850 Pneumatic PicoPump with Hold Pressure
- PV830 Pneumatic PicoPump with Hold Pressure and Vacuum
- uPump Pneumatic PicoPump with built in compressor
- MICRO-ePUMP with built in compressor and MICRO-ePORE™ Cell Penetrator to facilitate microinjections
- UMPIII UltraMicroPump
- Nanoliter2020 Injector
MANIPULATOR
PULLER
- PUL-1000 Programmable Pipette Puller
- SU-P30 Vertical Pipette Puller
- SU-P97 Flaming/Brown Pipette Puller
- SU-P1000 Micropipette Puller
ACCESSORIES
- 801566/801963 Vacuum Pump for use with the PV830
- NanoFil Microliter Syringes
- MicroFil for Backfilling Glass Needles
- MICRO-ePORE Cell Penetrator
- PRO-300HDS HD Camera with View Screen
- Z-LITE-186 Fiber optic illuminator with (500186) Bifrucate Light Guides
- FluoroDish Optical Grade Glass Bottom Cell Culture Dishes
- Glass Capillaries
- Z-MOLDS Microinjection and Transplantation Molds
- Pipetters
- MicroTip Pre-pulled Pipettes
- E2XX Micropipette Storage Jar
- 504134 LED Ring Light
- M10 Manipulator Stand
- M-3 Tiilting Manipulator Base
- Many Surgical Instruments, including:
 |
 |
 |
| The world's smallest dead volume (2.7µL) injection syringe system when the 10μL NanoFil syringe is used with WPI needles 33-36g. It comes with various needle sizes from 26 ga. to 36 ga. Versatile research applications are available, including RPE and IO Kits. Custom needle shapes are available —blunt, sharp, beveled. | UMP3 is a motorized syringe pump that accepts syringes from 0.5μL–1mL. Using a 10μL syringe, the actual, minimum volume is 5–25nL. For intuitive, intelligent control, the UMP3 is combined with the Micro4 controller. | Microprocessor-controlled Nanoliter Injector NANOLITER2020 with SMARTouch controller uses positive dis place ment injection, eliminating the need for pipette calibration. System uses glass micropipettes. |
References
Warmerdam, T., Schröder, F., Wit, H., & Albers, F. (n.d.). Perilymphatic and endolymphatic pressures during endolymphatic hydrops. European Archives of Oto-Rhino-Laryngology, 260(1), 9–11. http://doi.org/10.1007/s00405-002-0518-2
Wei, J., Song, J., Jiang, S., Zhang, G., Wheeler, D., Zhang, J., … Liu, R. (2017). Role of intratubular pressure during the ischemic phase in acute kidney injury. American Journal of Physiology - Renal Physiology, 312(6), F1158–F1165. http://doi.org/10.1152/ajprenal.00527.2016
Petrie, R. J., Koo, H., & Yamada, K. M. (2014). Generation of compartmentalized pressure by a nuclear piston governs cell motility in a 3D matrix. Science, 345(6200), 1062–1065. http://doi.org/10.1126/science.1256965
Petrie, R. J., Koo, H., & Yamada, K. M. (2014). Generation of compartmentalized pressure by a nuclear piston governs cell motility in a 3D matrix. Science (New York, N.Y.), 345(6200), 1062–5. http://doi.org/10.1126/science.1256965
Petrie, R. J., & Koo, H. (2014). Direct measurement of intracellular pressure. Current Protocols in Cell Biology / Editorial Board, Juan S. Bonifacino ... [et Al.], 63, 12.9.1-9. http://doi.org/10.1002/0471143030.cb1209s63
Pacella, J. J., Kameneva, M. V, Brands, J., Lipowsky, H. H., Vink, H., Lavery, L. L., & Villanueva, F. S. (2012). Modulation of pre-capillary arteriolar pressure with drag-reducing polymers: a novel method for enhancing microvascular perfusion. Microcirculation (New York, N.Y. : 1994), 19(7), 580–5. http://doi.org/10.1111/j.1549-8719.2012.00190.x
Park, J. J.-H., Boeven, J. J., Vogel, S., Leonhardt, S., Wit, H. P., & Westhofen, M. (2012). Hydrostatic fluid pressure in the vestibular organ of the guinea pig. European Archives of Oto-Rhino-Laryngology, 269(7), 1755–1758. http://doi.org/10.1007/s00405-011-1813-6
Hepatic microvascular pressure during anaphylactic shock in anesthetized rats. (2009). Microvascular Research, 78(2), 169–173. http://doi.org/10.1016/J.MVR.2009.06.007
Valk, W. L., Wit, H. P., & Albers, F. W. J. (2006). Rupture of Reissner’s membrane during acute endolymphatic hydrops in the guinea pig: a model for Ménière’s disease? Acta Oto-Laryngologica, 126(10), 1030–1035. http://doi.org/10.1080/00016480600621722
Kopp, R., Schwerte, T., & Pelster, B. (2005). Cardiac performance in the zebrafish breakdance mutant. The Journal of Experimental Biology, 208(Pt 11), 2123–34. http://doi.org/10.1242/jeb.01620
Lucitti, J. L., Tobita, K., & Keller, B. B. (2005). Arterial hemodynamics and mechanical properties after circulatory intervention in the chick embryo. The Journal of Experimental Biology, 208, 1877–1885. http://doi.org/10.1242/jeb.01574
Understanding cardiovascular physiology in zebrafish and Xenopus larvae: the use of microtechniques. (2003). Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 135(1), 131–145. http://doi.org/10.1016/S1095-6433(03)00044-8
Hu, N., Yost, H. J., & Clark, E. B. (2001). Cardiac morphology and blood pressure in the adult zebrafish. The Anatomical Record, 264(1), 1–12. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11505366
Ishii, T., Kuwaki, T., Masuda, Y., & Fukuda, Y. (2001). Postnatal development of blood pressure and baroreflex in mice. Autonomic Neuroscience : Basic & Clinical, 94(1–2), 34–41. http://doi.org/10.1016/S1566-0702(01)00339-3
Hu, N., Sedmera, D., Yost, H. J., & Clark, E. B. (2000). Structure and function of the developing zebrafish heart. The Anatomical Record, 260(2), 148–57. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10993952
Kelly, S. M., & Macklem, P. T. (1991). Direct measurement of intracellular pressure. The American Journal of Physiology, 260(June), C652–C657. http://doi.org/10.1002/0471143030.cb1209s63
Proximodistal gradient in endoneurial fluid pressure. (1988). Experimental Neurology, 102(3), 368–370. http://doi.org/10.1016/0014-4886(88)90233-6
Tanner, C., Frambach, D. A., & Misfeldt, D. S. (1983). Transepithelial transport in cell culture. A theoretical and experimental analysis of the biophysical properties of domes. Biophysical Journal, 43(2), 183–90. http://doi.org/10.1016/S0006-3495(83)84339-2
Rabito, C. A., Tchao, R., Valentich, J., & Leighton, J. (1980). Effect of cell-substratum interaction on hemicyst formation by MDCK cells. In Vitro, 16(6), 461–8. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6248454

Close