This website uses cookies to ensure you get the best experience on our website.
Read more
APP NOTE: Observation of Mitosis using EVOM™ AutoLCI
March 03, 2023

Introduction
 In the process of ‘cell cycle’, cells grow and divide into two genetically identical daughter cells. It is regulated by a complex signaling pathway which keeps cell homeostasis by regulating cell division and DNA duplication1. On the other hand, because cancer cells grow and divide indefinitely out of cell cycle control, anti-mitotic drugs are used to suppress abnormal proliferation of cancer cells2. In particular, Nocodazole is known to be a representative anti-mitotic drug for cancer treatment, and it has the characteristics of disturbing microtubule dynamics during cytoplasmic and nuclear division3,4.
In the process of ‘cell cycle’, cells grow and divide into two genetically identical daughter cells. It is regulated by a complex signaling pathway which keeps cell homeostasis by regulating cell division and DNA duplication1. On the other hand, because cancer cells grow and divide indefinitely out of cell cycle control, anti-mitotic drugs are used to suppress abnormal proliferation of cancer cells2. In particular, Nocodazole is known to be a representative anti-mitotic drug for cancer treatment, and it has the characteristics of disturbing microtubule dynamics during cytoplasmic and nuclear division3,4.
In the present study, we examined the anti-mitotic activity of nocodazole against cancer cell line by monitoring the cell division process. To this end, Hela cells were stably transfected with green fluorescent protein fused histone 2B (H2B-GFP), which visualizes the dynamics of chromosomal structure during cell cycle progression5. And then, the cell division was monitored in real time after treating the cell with or without nocodazole using EVOM™ AutoLCI, a live cell imaging device.
Method
HeLa cells transfected with H2B-GFP plasmid were seeded into a 24 well plate at 4 x 104 cells/well. Cells were cultured with 10% Fetal bovine serum and Dulbecco’s Modified Eagle’s Medium to which 300 ug/ mL G418 was added. After overnight culture to attach the cells, they were incubated with or without 62.5 nM of nocodazole, and real time imaging was conducted using EVOM™ AutoLCI (Bright field & green fluorescence channel, 10X optics). Images were taken every 15 minutes for 24 hours.
Results
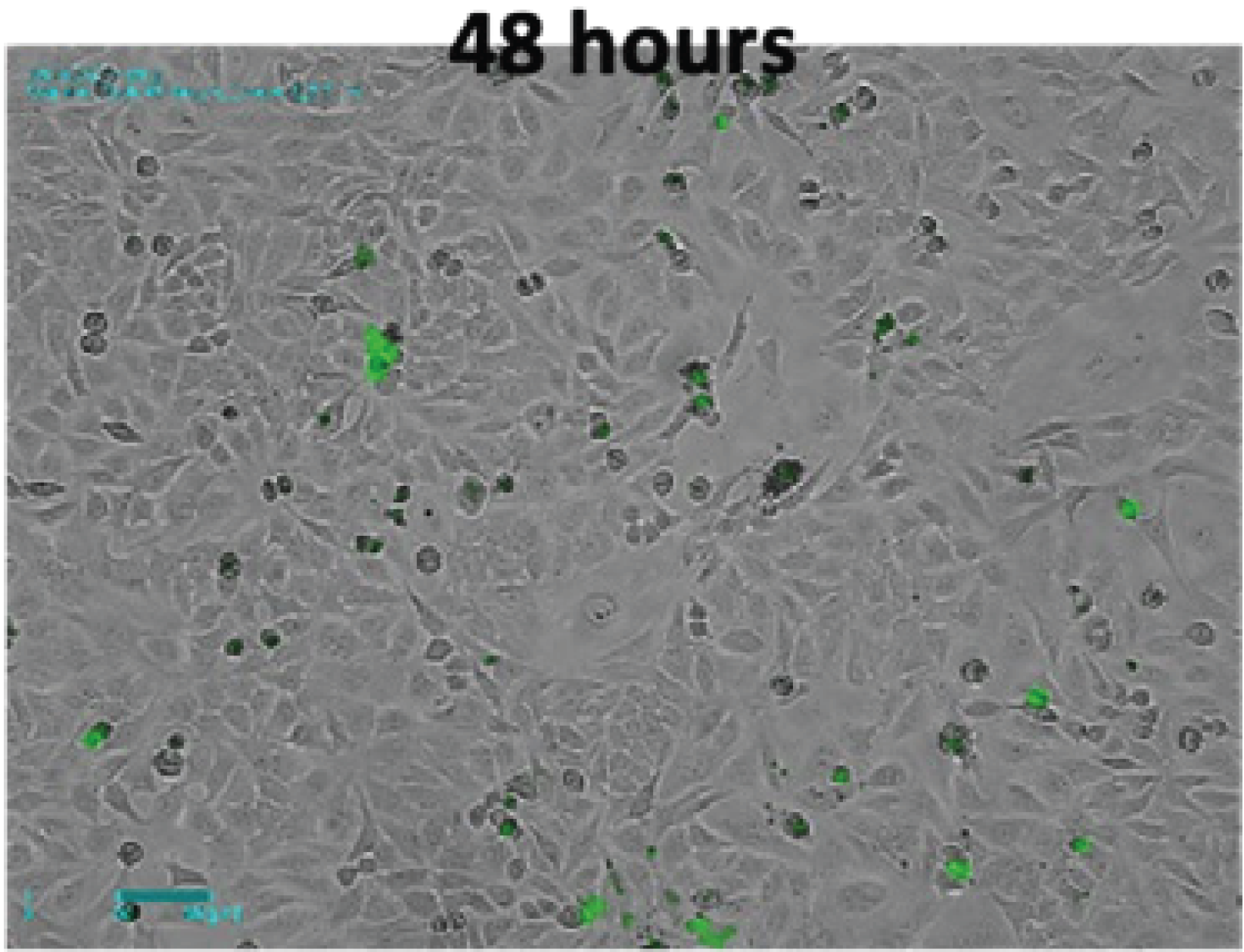
Brightfield and fluorescence time-lapse imaging of Hela cells transfected with green fluorescent protein fused H2B was performed to observe nuclear changes in mitosis. In the bright field image, the nuclear membrane was clearly visible at the beginning, then they gradually disappeared and metaphase where chromosomes were aligned at the center of the cell was observed in both the bright field and fluorescence images (Figure 1A). At this point, chromosomes were condensed, and fluorescence became more intense compared to the previous stage. Chromosomes that entered the later stage were separated into two sister chromatids and each one moved to opposite poles of the cell. And as shown in the last image, two daughter cells were finally generated in telophase. On the contrary, in the cells treated with nocodazole, the cell division process did not proceed any further as the chromatids could not be divided into both ends. In the bright field image, the cells continued to not adhere to the bottom anymore, and it was confirmed that the DNA was eventually fragmented, which resulted in apoptosis in the fluorescence image (Figure 1B).
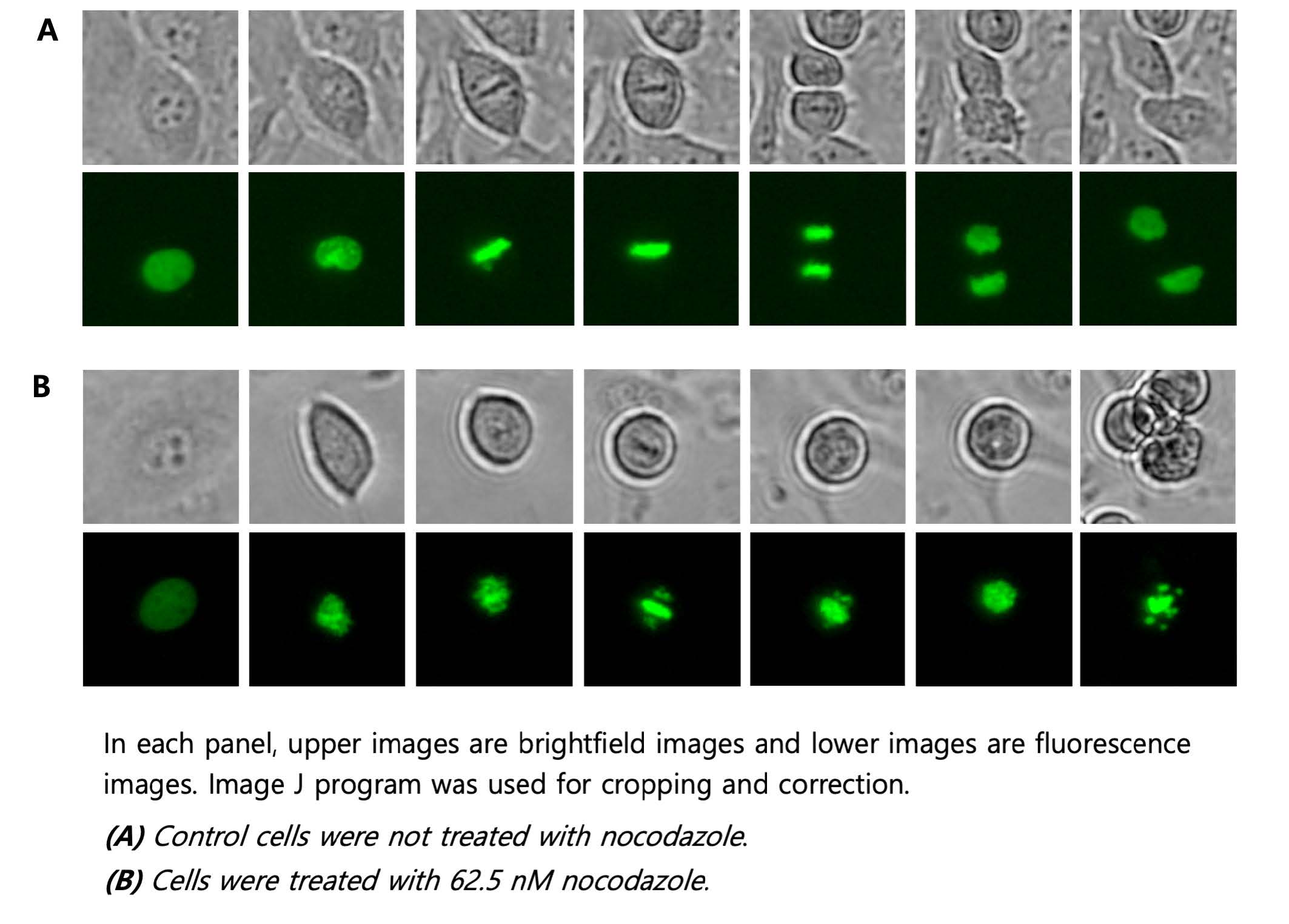
Figure 1. Timelapse images of H2B-GFP transfected Hela cells with or without nocodazole.
Conclusion
Fluorescent labeling is a useful tool to observe the dynamics of organelles in live cells. So far, plasmids have been developed to observe various organelles or target protein using fluorescent fusion protein, and many studies have been conducted to verify the changes in organelles or protein translocation induced by various stimulations.
In this application note, we performed a nuclear labeling method using histone and green fluorescence fusion protein that binds to DNA. To confirm the effect of nocodazole on cell division, cells were monitored over time and images were acquired using EVOM™ AutoLCI, a real-time live cell imaging device. The system has a fully motorized camera that enables imaging of various positions at a set interval programmed by the user. This makes it possible to track changes in each cell under different conditions.
References
- Mills, Christopher C., E. A. Kolb, and Valerie B. Sampson. "Development of Chemotherapy with Cell-Cycle Inhibitors for Adult and Pediatric Cancer Therapy Combination Therapies for Cancer." Cancer research 78.2 (2018): 320-325.
- Otto, Tobias, and Piotr Sicinski. "Cell cycle proteins as promising targets in cancer therapy." Nature Reviews Cancer 17.2 (2017): 93-115.
- Blagosklonny, Mikhail V. "The power of chemotherapeutic engineering: arresting cell cycle and suppressing senescence to protect from mitotic inhibitors." Cell cycle 10.14 (2011): 2295-2298.4. Taciak, Bartłomiej, et al. (2018) PloS one
- Endo, Kingo, et al. "Nocodazole induces mitotic cell death with apoptotic-like features in Saccharomyces cerevisiae." FEBS letters 584.11 (2010): 2387-2392.
- Anda, Teru, Kevin F. Sullivan, and Geoffrey M. Wahl. "Histone–GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells." Current Biology 8.7 (1998): 377-385.
FOR RESEARCH USE ONLY and not for use in diagnostic procedures. Copyright © 2022, by CURIOSIS Inc. All rights reserved.

Close