This website uses cookies to ensure you get the best experience on our website.
Read more
APP NOTE: Analysis of Nocodazole-Induced Cytotoxicity Using EVOM™ AutoLCI
March 01, 2023
Introduction
Cytotoxicity refers to the degree of damage to cells.  caused by chemical substances or physical factors. Measuring it through cytotoxicity assay is essential for drug development and biological research. Cells undergo complex signaling pathways that causes various cell death processes such as apoptosis, necrosis, and necroptosis. However, most cytotoxicity assays are measured at the endpoint that makes it difficult to study the dynamic response of cells to drugs.
caused by chemical substances or physical factors. Measuring it through cytotoxicity assay is essential for drug development and biological research. Cells undergo complex signaling pathways that causes various cell death processes such as apoptosis, necrosis, and necroptosis. However, most cytotoxicity assays are measured at the endpoint that makes it difficult to study the dynamic response of cells to drugs.
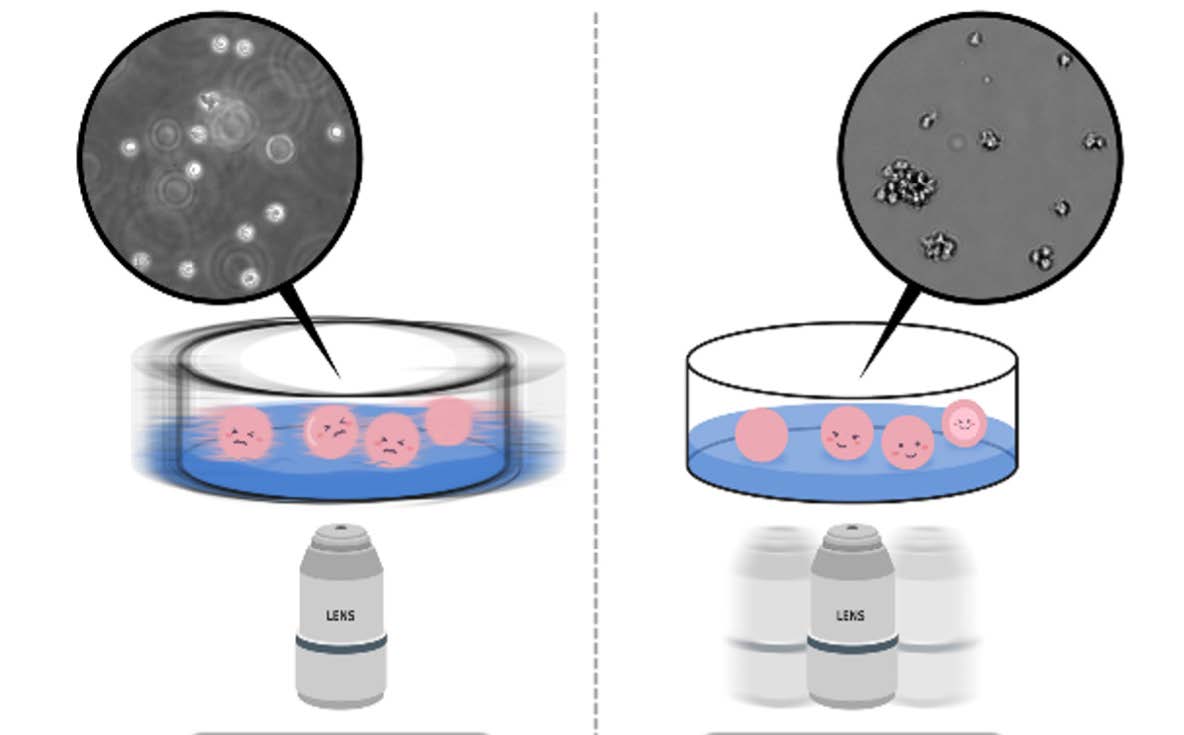
In this application note, we aimed to examine the performance of a cytotoxicity assay using real-time imaging. Cells treated with various concentrations of Nocodazole, the anti-cancer drug, were stained with fluorescent dye during cell death, then monitored with EVOM™ AutoLCI. It was observed through time-lapse imaging that apoptosis increased in a Nocodazole dose-dependent manner, and the degree of apoptosis was quantitatively measured and graphed using the Analysis software provided with the EVOM™ AutoLCI.
Method
HeLa cells were counted using Facscope B, an automatic cell counter and seeded at 1 x 104 cells/well in a 48 well plate. After culturing overnight, cells were treated with various concentrations (16.625 nM, 31.25 nM, 62.5 nM, 125 nM, 250 nM) of Nocodazole. At this point, CellTox green dye (Promega, G8742) which binds to DNA of cells with impaired membrane integrity during cell death was added to the sample. Using EVOM™ AutoLCI installed inside an incubator, cell images were acquired every 1 hour for 48 hours then the images were analyzed using the Analysis software. The cell death rate (%) was calculated as fluorescence coverage (= dead cell) ÷ bright field confluency (= total cell).
Results
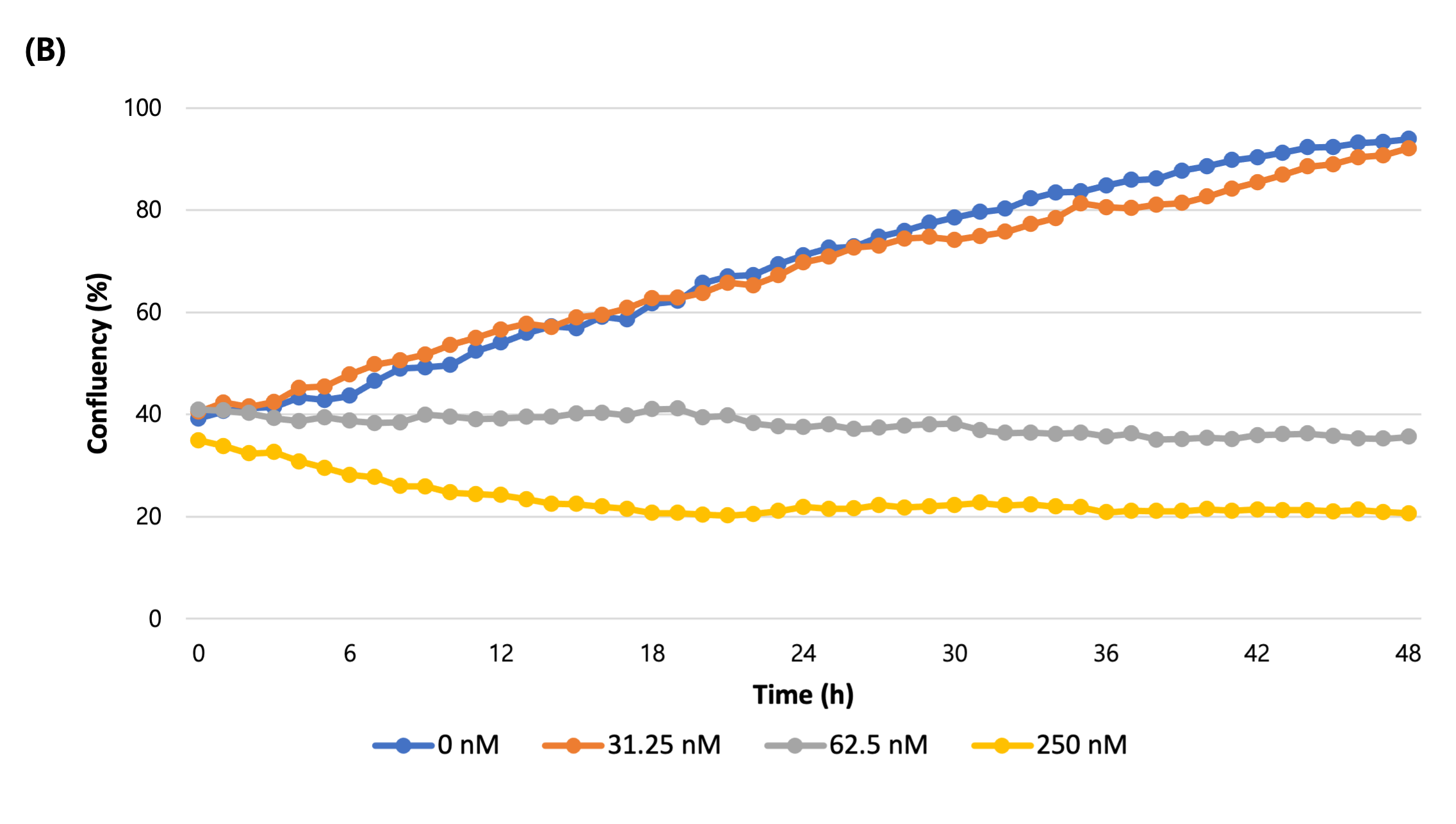
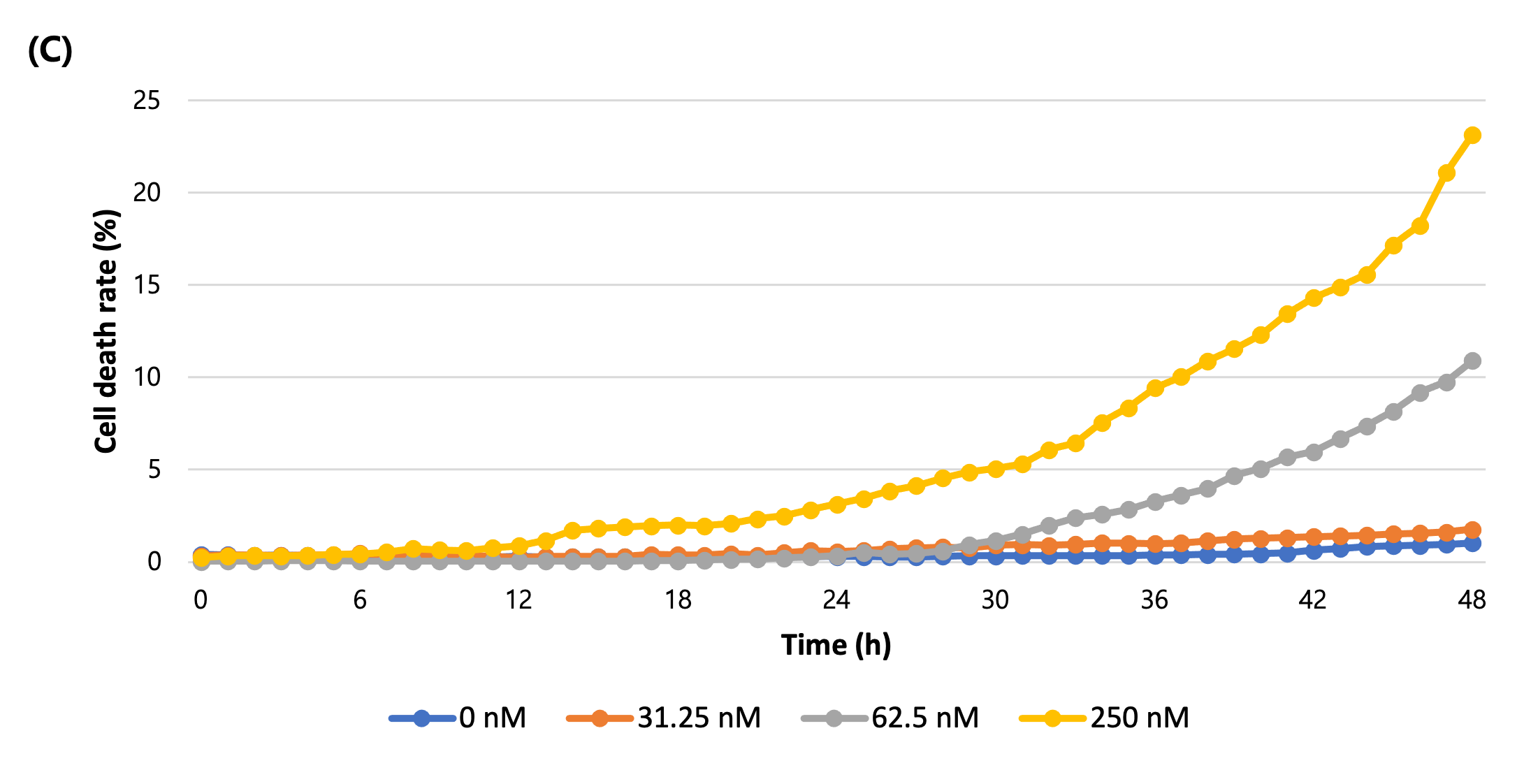
As a result of treating the cells with nocodazole by different concentrations and imaging them over time, the morphology of cells changed in a concentration-dependent manner and the confluency shrinkage was observed in bright field imaging (Figure 1A, B). In addition, fluorescence imaging of dead cells stained by CellTox dye, measuring its coverage and quantifying the cell death showed that the proportion of fluorescent cells increases with apoptosis (Figure 1C).
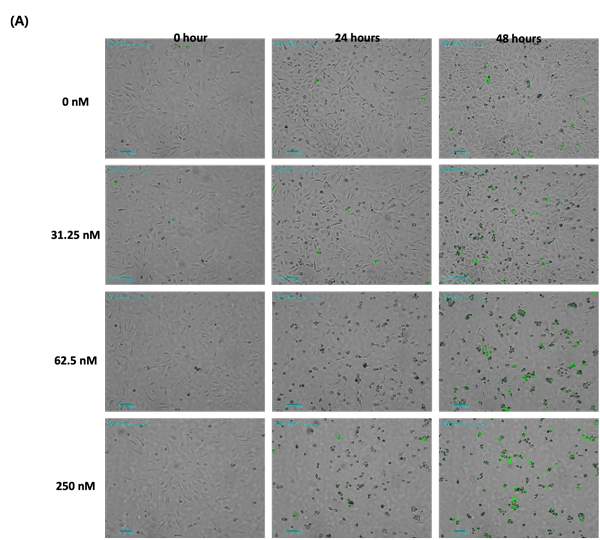
Figure 1. Analysis of Nocodazole dose-dependent cell death using time-lapse imaging
(A) Merged bright field and fluorescence images for each concentration of Nocodazole.
The images are shown at 24-hour intervals, and green fluorescence indicates the dead cells. (Scale bar: 200 um)
(B) The confluency (%) graph of total cell over time.
(C) The graph of cell death rate (%) over time.
Conclusion
EVOM™ AutoLCI is a live cell imaging device that can simultaneously perform bright field and fluorescence imaging in an incubator. The system has a fully motorized camera that enables imaging of various positions at a set interval programmed by the user. The confluency graph over time can be acquired by calculating the confluency of captured images using the Analysis software. Furthermore, by measuring the fluorescence coverage, the degree of apoptosis according to the concentration of Nocodazole can be quantified.
FOR RESEARCH USE ONLY and not for use in diagnostic procedures. Copyright © 2022, by CURIOSIS Inc. All rights reserved.

Close