This website uses cookies to ensure you get the best experience on our website.
Read more
APP NOTE: Common Applications of the EVOM™ Auto System
May 01, 2023

Improved User Experience
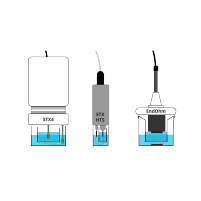
 EVOM™ Auto is the latest generation of WPI’s automated transepithelial or transendothelial electrical resistance (TEER) measurement system. Using the same proven technology in the EVOM™ Manual and REMS, combined with a new multi-electrode array, software interface and control system, it delivers our fastest workflow solution while improving TEER measurement accuracy:
EVOM™ Auto is the latest generation of WPI’s automated transepithelial or transendothelial electrical resistance (TEER) measurement system. Using the same proven technology in the EVOM™ Manual and REMS, combined with a new multi-electrode array, software interface and control system, it delivers our fastest workflow solution while improving TEER measurement accuracy:
- Faster throughput – read a 96-well plate in under 3.5 minutes (A 2-rinse cycle can be completed in 7 minutes, almost halving the time compared to a REMS)
- Automatic sample averaging improves accuracy and stability
- Compact size for easy set up and operation inside in a hood or incubator
- Wireless control of the autosampler
- Smart user interface and web browser-based software for easy sample analysis and data storage and access
- Wider resistance range compared to the REMS – upper range increased from 20 kΩ to 100 kΩ
- Continuous data recording capability at user-defined time intervals
EVOM™ Auto, with advanced product features, is designed for a better user experience to analyze samples and increase sample reading throughput while acquiring more accurate TEER data.
In vitro Cell Culture Models
 The in vitro cell culture models of human endothelial and epithelial monolayers are considered as reliable models of the in vivo environments and as such are used for drug toxicity and transport studies. Findings of the in vitro models are also translated to ascertain the metabolic and physiological functions of a particular pharmacological entity. The most commonly used endothelial/ epithelilal in vitro models are:
The in vitro cell culture models of human endothelial and epithelial monolayers are considered as reliable models of the in vivo environments and as such are used for drug toxicity and transport studies. Findings of the in vitro models are also translated to ascertain the metabolic and physiological functions of a particular pharmacological entity. The most commonly used endothelial/ epithelilal in vitro models are:
- Blood-brain barrier (BBB) model
- Gastrointestinal tract (GIT) model
- Pulmonary models (including viral infection model, such as COVID-19)
TEER Measurement with EVOM™ Auto (HTS TEER Measurement System)
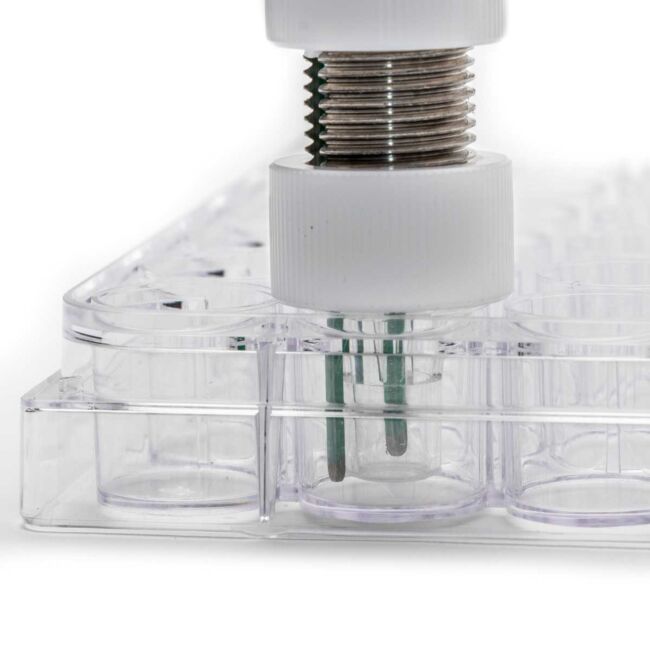
The EVOM™ Auto, offered by WPI, measures the electrical resistance of transepithelial/transendothelial cells grown to confluence
on semi-permeable microporous filters of high throughput screening (HTS) 96-well microplates. The system is automated, which minimizes errors associated with a user's manual handling, generating highly reliable data and enhanced reproducibility. The
system operation is controlled with an iPad tablet and a local wireless network. Automated measurement of tissue resistance in
high throughput (HTS) transwell plates provides the critical advantages of speed, precision, decreased chances of introducing contamination, and the instant availability of measured resistance or TEER data. These measurements are useful in applications, such as evaluating drug toxicity, and bioavailability studies.
Drug Discovery/Toxicology
The TEER method is considered fast, accurate and non-invasive, the prime choice of all the available methods. Notably, for evaluating pharmacological transport across these barriers, this method does not add any additional molecule or chemical in the system. The EVOMTM Auto has the capability to measure the electrical resistance of transepithelial/transendothelial cellular layers growing to confluence on microporous filters. For high throughput screening (HTS), the instrument has the versatility for use in 96-well HTS transwell plates.
Conclusions
The TEER measurements in the in vitro endothelial/epithelial barrier model (like the blood-brain barrier models, gastrointestinal tract models, and pulmonary models) can be efficiently used to evaluate pharmacological toxicities, as well as have applications in the drug discovery process. WPI's new EVOM™ Auto design includes wireless autosampler control, smart user interface, compact autosampler size, and advanced measurement electronics. EVOM™ Auto, with advanced product features, simplifies and improves throughput and measurement accuracy of automated TEER screening of the in vitro tissue models.
References
- Srinivasan, B., et al., TEER measurement techniques for in vitro barrier model systems. J Lab Autom, 2015. 20(2): p. 107-26.
- Henry, O.Y.F., et al., Organs-on-chips with integrated electrodes for trans-epithelial electrical resistance (TEER) measurements of human epithelial barrier function. Lab Chip, 2017. 17(13): p. 2264-2271.
- D'Souza, W.N., et al., Differing roles for short chain fatty acids and GPR43 agonism in the regulation of intestinal barrier function and immune responses. PLoS One, 2017. 12(7): p. e0180190.
- Mukhtar, M. and R.J. Pomerantz, Development of an in vitro blood-brain barrier model to study molecular neuropathogenesis andneurovirologic disorders induced by human immunodeficiency virus type 1 infection. J Hum Virol, 2000. 3(6): p. 324-34.
- Noda, S., S. Tanabe, and T. Suzuki, Differential effects of flavonoids on barrier integrity in human intestinal Caco-2 cells. J Agric Food Chem,2012. 60(18): p. 4628-33.
- Dekali, S., et al., Assessment of an in vitro model of pulmonary barrier to study the translocation of nanoparticles. Toxicol Rep, 2014. 1: p.157-171.
- Shityakov, S., et al., Sevoflurane-Sulfobutylether-beta-Cyclodextrin Complex: Preparation, Characterization, Cellular Toxicity, MolecularModeling and Blood-Brain Barrier Transport Studies. Molecules, 2015. 20(6): p. 10264-79.
- Rayner, R. E., Makena, P., Prasad, G. L., & Cormet-Boyaka, E. (2019). Optimization of normal human bronchial epithelial (NHBE) cell 3Dcultures for in vitro lung model studies. Scientific reports, 9(1), 500.

Close