This website uses cookies to ensure you get the best experience on our website.
Read more
EVOM™ Epithelial Volt/Ohm (TEER) Meter 3 - DISCONTINUED
$3,300.00
Prices valid in USA, Canada, and PR only.
Order code
EVOM3

Prices valid in USA, Canada, and PR only.
This product has been discontinued. UPGRADE TO EVOM™ MANUAL for increased accuracy, auto data logging, touchscreen interface, and many new features.
EVOM™ Manual replaces all WPI-manufactured manual TEER meters, including EVOM3, EVOM2 and MilliCell® ERS-2, all of which have been discontinued.
Prices valid in USA, Canada, and PR only.
TEER measurement with automatic data logging
- Low noise design offers greater resolution and accuracy
- Automatic 20X sample averaging improves accuracy and stability
- Adjustable fixed measurement currents (2, 4 or 10 μA)
- Resistance auto ranging from 1 Ω to 100,000 Ω or with three fixed current ranges
- Reliable low current, low voltage design prevents metal ion transport
- Fast resistance stabilization on low levels under 200 Ω with resolution to 0.1 Ω
- Ergonomic tilt stand for low glare operation
- Graphical display of popular plates (6, 12, 24, 96) for trend analysis
- Display shows the most recent set of parameters
- Automatic plate indexing operation with or without control well subtraction for resistance and potential difference (PD) measurements
- Continuous data logging via USB (PC, Mac, Linux)
- Saves date stamped data to a spreadsheet readable file on a USB drive
- Upgradable firmware
Next Generation Epithelial Volt Meter
EVOM3 Monitors Cellular Health
WPI's EVOM system is popular in the research community, and it is commonly used for the evaluation of mammalian cellular health by measuring transepithelial/transendothelial electrical resistance (TEER or TER) of cellular layers.
EVOM3 works on the same basic principle as older EVOM models (EVOMX, EVOM and EVOM2). It has advanced features for performing experiments more easily. With the new touchscreen display you can now STORE DATA as Microsoft® Excel files on a USB flash drive. Just remove the flash drive with all your recorded data from the EVOM3 and plug it into a computer to access and plot your data. It is as simple as it sounds.
TEER: Transcellular & Paracellular Pathway of Ion or Electrical Current Flow
Ions and electrical current can be transported through the cells (transcellular) and through the space between adjacent cells (paracellular) as depicted in the image below.
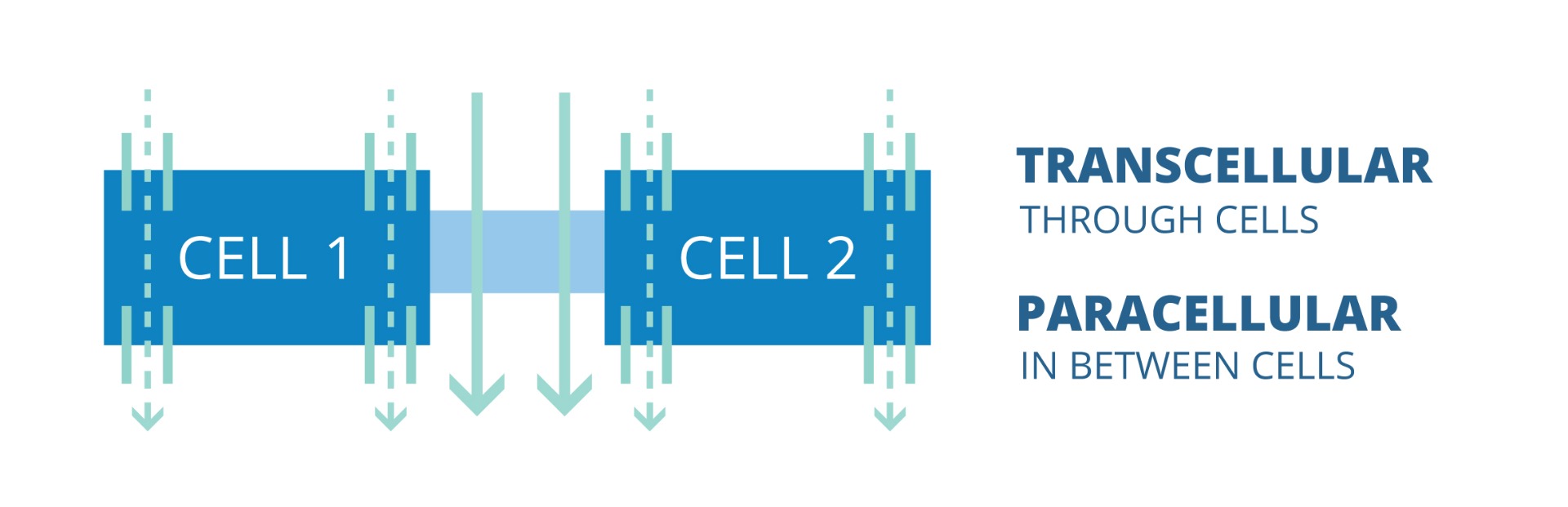
The dotted lines show the transcellular pathway of ion or electrical current flow. The solid lines demonstrate the paracellular pathway of ion or current flow.
EVOM3 TEER Measurement Basic Working Principle
Electrical resistance (i.e., TEER) of a cellular layer is the inverse presentation of the electrical conductance through the cellular layer. A high TEER value of the cellular layer is indicative of an intact cellular monolayer and suggests low or restricted permeability of ions and molecules (i.e., low conductance). Similarly, a decrease in the TEER value suggests a compromised barrier function and indicates increased permeability. Tissue permeability studies require a confluent cellular layer, and TEER measurement is gennerally used to confirm the formation of a confluent monolayer.
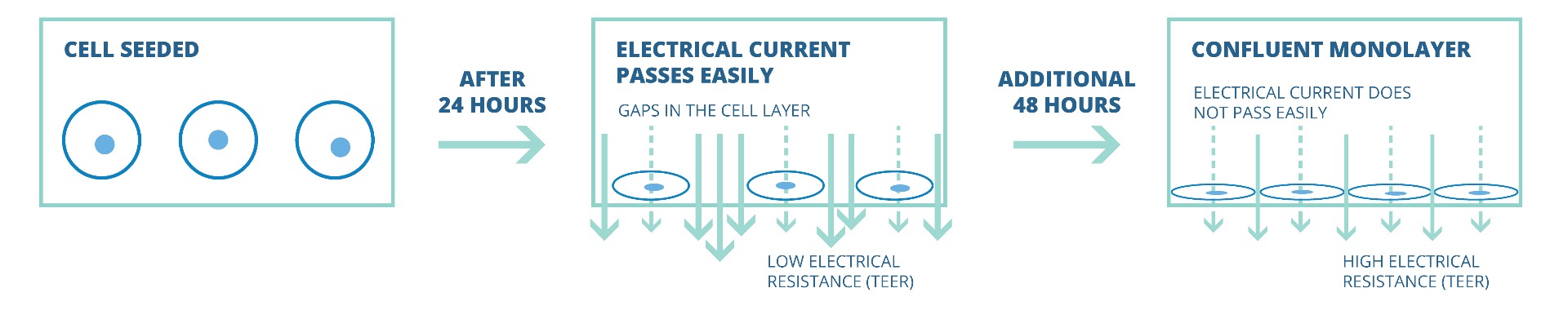
Initially, 24 hours after cells eeding transwell, TEER values are generally low, because the current passes can pass easily between the cells. Over time, the cells multiply and start covering the gaps. Finally, a confluent cellular monolayer is formed. At that point the permeable membrane is fully covered with cells and does not allow easy passage of electrical current. This results in a high TEER value.
TEER Measurement of Leaky & Tight Cell Types
TEER values of confluent cellular monolayers can vary depending on the cell type. Monolayers of certain cell types (e.g., cell type A), which normally show low TEER values, generally have relatively leaky tight junctions. Monolayers of other cell types (e.g., cell type B) show high TEER values, and these cell types are known to have tight tight junctions. Ions and molecules are known to pass rather easily across leaky cellular layers as compared to tighter cellular layers.The presence of more transcellular ion channels on cells can further allow easier flow of ions or electrical current through the transcellular pathway, which can additionally lower TEER values.
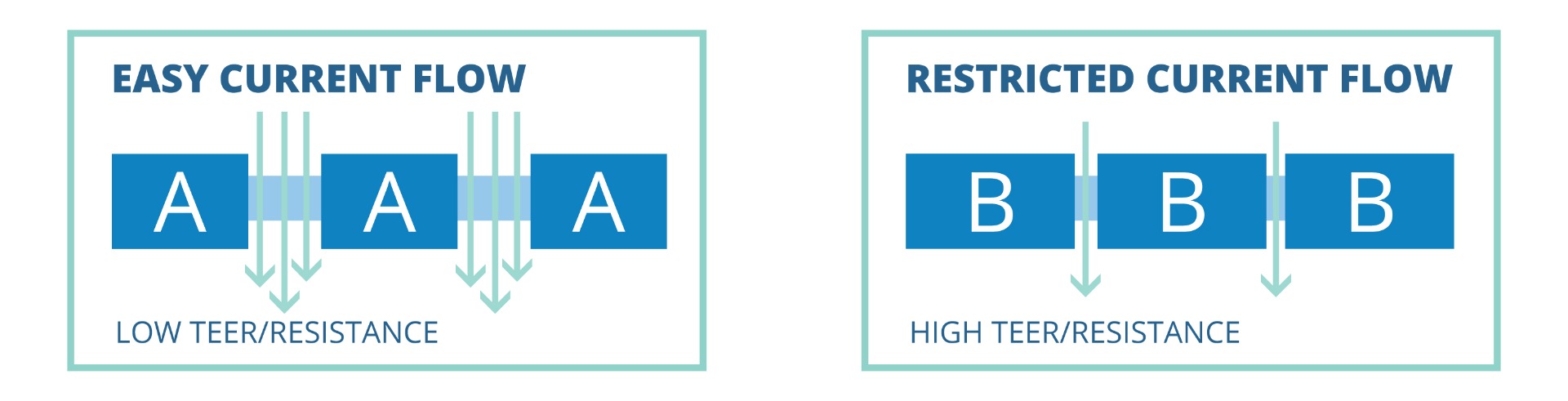
Cell type A allows greater amounts of current and ions to pass between the cells and yields a low TEER value. With its tighter junctions, cell monolayers of type B cells will show a higher TEER value. While both monolayers are confluent, the TEER resistance values can be markedly different based on the nature of the cells themselves.
Why Choose WPI's EVOM System?
WPI was the pioneer in introducing the simplified TEER measurement technique using EVOM, and to date WPI's EVOM system remains the most popular device for measuring TEER values in transwells. The EVOM3 is the newest version of epithelial volt meters, with several advanced features. The EVOM3 has a touchscreen interface that makes it simple to use. TEER measurement using an EVOM is a non-invasive method of monitoring cellular health. The EVOM3 with the new STX2PLUS electrode offers more accurate sample analysis and quick, simple data storage features using a USB flash drive.
![]()
For quantitative sample analysis with higher accuracy and easy data storage, consider EVOM3. The non-invasive method of EVOM3 detection allows the same sample to be used for other experimental analyses.
EVOM3 TEER: Key Applications
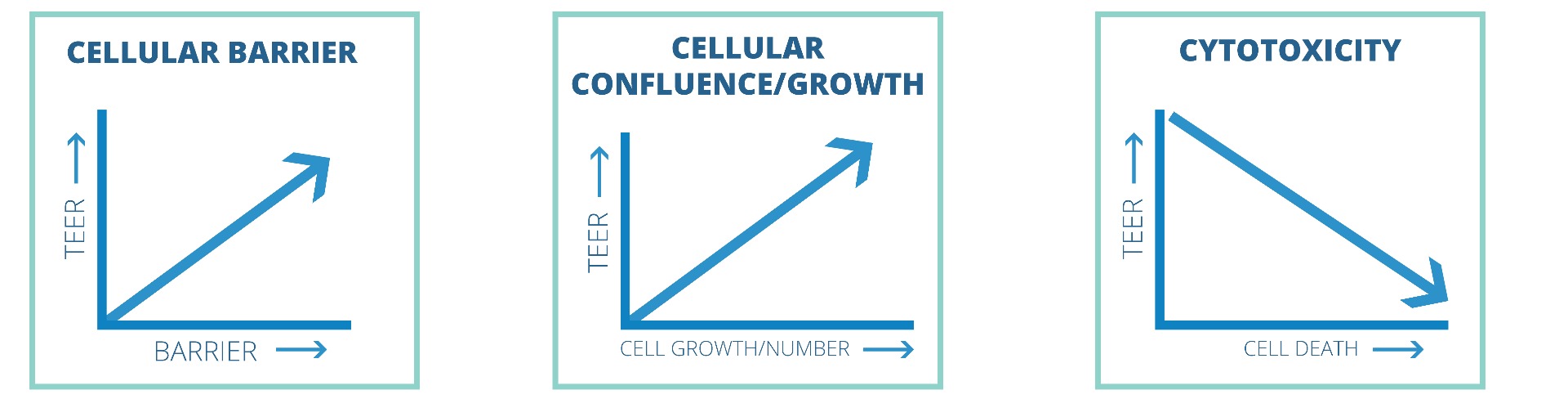
Here are three applications where TEER measurement is commonly used. When measuring the cellular barrier function, the rise of TEER values generally correlates with increased barrier function. Similarily, elevation of the TEER value to the maximul level can indicate that the cellular layer has reached confluence. Cellular cytotoxicity can be evaluated by measuring TEER. High TEER values indicate a healthier cellular layer. As the cells die, gaps in the cellular layer can form, and the TEER value can drop.
EVOM3 TEER: Emerging Application Fields
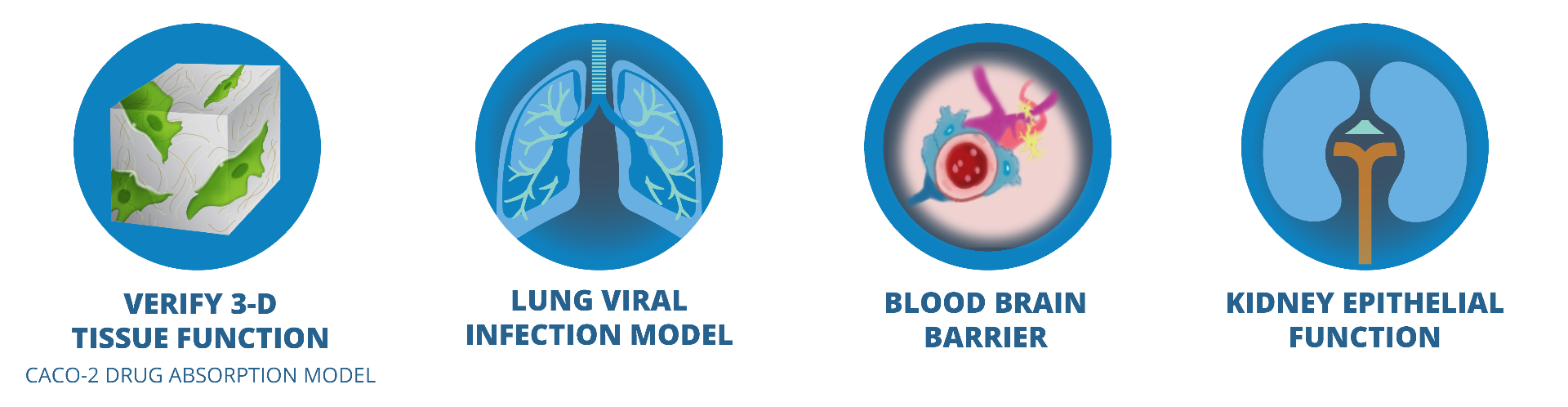
WPI's EVOM system has been extensively used to study in vitro 2-dimensional (2-D) or 3-D tissue health and function. In recent years, for high throughput drug screening and to study diseases, more research fucus has been given in creating 3-D in vitro tissues that resemble in vivo tissues and show consistent functional properties. TEER Measurement is used as one of the methods to evaluate and compare how closely in vitro tissues can mimic in vivo tissues consistently. EVOM3 can be used in 3-D in vitro models, such as the Blood Brain Barrier (BBB), Ling Virus Infection, and Intestine, Kidney and Liver tissues. The References section lists a few selected publications.
VIDEOS
Short videos highlight some of the key new features of the EVOM3. Click on the VIDEOS tab on this page to see all the EVOM3 videos.
More on Epithelial Physiology
WPI offers a line of products for the study of epithelial physiology including a variety of electrodes, the EVOM meters and the automated robotic system for high throughput screening (HTS). Get the details on the electrode options in the article "How To Select Electrodes For Making TEER Measurements."
Benefits
- Eliminates errors and reduces experimental processing time
- Auto data logging eliminates the need to track data by hand
- The small footprint allows more bench space
- Easy calibration and verification
- Footswitch for hands-free recording
- Prevent data loss with auto save and data recovery when battery is low
- TEER is easily computed by applying a unit area formula to the resistance
Applications
- Measure epithelial or endothelial tissues for confluence, TEER and potential difference
- Permeability, conductance and drug studies
- Continuous digital monitoring of a target membrane
- Common studies
- Blood-brain barrier transport
- Lung epithelial tissue studies
- Intestinal tissue studies
- Skin studies
EVOM3 For Teer Measurement
The EVOM3 delivers improved workflow efficiency, more stable and repeatable measurements versus traditional Trans Epithelial Electrical Resistance (TEER) meters. Providing users with vital feedback during experiment measurements, the EVOM3’s large screen offers a range of informational views. The new graphical displays for trend analysis and measurement values helps scientists deliver simple, stepwise methodology during experimental measurements. The touch screen interface provides users an intuitive, easy-to-use menu for configuration.
Eliminating the need to log data by hand, the EVOM3 writes the resistance or voltage information to a USB drive in CSV format for easy transfer to spreadsheets and data analysis programs. When used with the footswitch it enables handsfree recording of measurements.
At the heart of the EVOM3 is our latest processor and circuitry, providing users with quick, easy and reliable readings due to its fast stabilization, automatic twenty times sampling average and low noise design. The auto ranging resistance feature allows for fast resistance measurements, and an over-range display feature eliminates false readings. The EVOM3 has adjustable current levels in three fixed ranges with two lower ranges for sensitive membranes and high resistance ranges up to 100 KΩ.
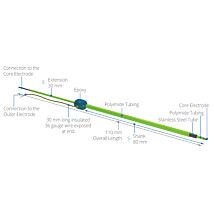
TEER Measurement Electrode
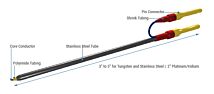
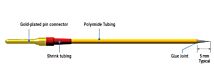
The STX2-PLUS electrode was designed for easy insertion into many 24-well plates. It is location re-placeable in the insert for repeatable and consistent measurements. The new shielded electrodes are now designed to minimize electrical interference and to be more easily maintained.
- STX2-PLUS new electrode designed for 12 and 24-well plates.
- Weighted self-standing electrode for hands-free stable measurements
- Shielded cable to minimize electrical and cell phone interference
System Components
| What is included with the EVOM3 | QTY |
| EVOM3 Epithelial Volt Ohm Meter | 1 |
| STX2-PLUS Electrode set | 1 |
| 300749 USB drive 32 GB (Used for storage. Also contains a Python 3.8 program for continuous digital monitoring of a target insert). | 1 |
| 503535 USB cable | 1 |
| 99673 Calibration kit, 1000Ω Test Resistor | 1 |
| 803025 A/C power cord and charger | 1 |
| 13142 Foot switch | 1 |
NOTE: A 99672 EVOM2 to EVOM3 Electrode Adapter is sold separately. The STX2, STX3 and all STX100s require the use of this adapter with the EVOM3.

STX2-Plus
STX2-Plus Benefits
- Keyed electrode base for repeatable placement gives more consistent results, eliminating the need for multiple readings.
- Easy to maintain


How Does the EVOM3 Work?
Confluence of a cellular monolayer is determined by an increase or a plateau in tissue resistance detected using the unique electronic circuit of the EVOM3 and the new STX2-PLUS electrode. The EVOM3 qualitatively measures cell monolayer health and quantitatively measures cell confluence. The EVOM3 produces a low AC current that avoids electrode metal deposits and adverse effects on tissues which can otherwise be caused by higher DC currents. The EVOM3 uses low current and voltages and is designed for non-destructive testing for epithelial monolayer confluence in cell cultures. In addition, resistance readings are unaffected by membrane capacitance or membrane voltage. The accuracy and repeatability of the EVOM3-STX2-PLUS system makes this instrument ideal for permeability, PD and other detailed membrane studies.
Electrodes For TEER (Epithelial) Measurement
| Part # | Descriptions |
| STX2-PLUS | Replacement Electrode Set |
| STX2* | Replacement Electrode Set (Requires 99672 for use with the EVOM3) |
| STX3* | Adjustable electrode set for shallow wells, 5-9 mm depth |
| 3993* | 2 mm Adapter for EVOM2 |
*(Requires 99672 for use with the EVOM3)
ENDOHM Chambers For Endothelial/Epithelial Measurement
NEW EndOhm chambers include the EVOM3 cable 99916.
| Part # | Descriptions |
| ENDOHM-6G | EndOhm for 6 mm culture cup (24 wells per plate) |
| ENDOHM-12G | EndOhm for 12 mm culture cup (12 wells per plate) |
| ENDOHM-24G | EndOhm for 24 mm and Costar Snapwell cup (6 wells per plate) |
TEER measurement techniques for in vitro barrier model systems
| SKU | EVOM3 |
|---|
Upsell Products
- $11.00
- $460.00
- $65.00
-
USB Thumb Drive for EVOM Manual, 32GB
As low as $15.00 - $227.00
Software for EVOM3
EVOM3 firmware upgrade package EVOM3 Rev 1.5 (current release)
FAQ
- Will the EVOM3 work with Endohm’s?
Yes, but the 99672 adaptor is required or the new EVOM3 cable 99916.
- Why would I want to use the blank function?
The blank feature is used when you want to subtract out any measurement that is not from the membrane, such as the electrode and fluid resistances.
- Does the EVOM3 system automatically calculate TEER?
No, TEER measurement requires an area calculation. To compute TEER, multiply the measured resistance by the appropriate surface area (below). For example, a 12 mm insert measures 565 Ω, the TEER is 565 Ω × 1.13 cm2 = 638.5 Ω- cm2. Here are the surface areas generally applicable to different transwell/insert formats: 6 well plate (24 mm inserts) 4.52 cm2, 12 well plate (12 mm inserts) 1.13 cm2, 24 well plate (6.5 mm inserts) 0.33 cm2, 96 well plate (4.3 mm inserts) 0.14 cm2.
- EVOM3 data is stored automatically when the last well is reached. How do I store the data when I only want to measure 8 of 96 wells?
Clear any data in memory by opening settings, store menu then press new plate, that will clear any prior readings. Return to the main screen, open the preview screen, select each well to measure (the selection turns green), place the electrode, then measure. When you’re done measuring the selected wells, open the settings, press the store screen menu, then press store new to save the plate data to the USB drive.
- How should you store the EVOM3 and electrodes if they will be exposed to UV light in a laminar hood for extended periods of time?
Take the EVOM3 out of the laminar hood after use. Next time, turn on the UV inside the hood. Once the hood is disinfected by UV, turn off UV, next spray 70-100% ethanol or isopropanol onto paper towel and wipe the EVOM3. Do not spray alcohol directly onto EVOM3.
- Why am I getting dashes as reading on EVOM3, even if I have the STX2-PLUS electrode inside the sample?
The electrode in the air or partially immersed in the liquid can show dashes since it records unstable read outs. The electrode tip portion (sensing region) must stay fully immersed. You may also notice unstable read outs when the electrode tip is not fully immersed. Make sure to select apical and basolateral volumes so that electrode tip stays fully immersed. You need to use apical and basolateral volumes greater than what is suggested by the insert manufacturer. For example, for Corning-24 well Transwell (example Corning 3470) we recommend minimum 300 µL on the top (apical) and 850 µL on the bottom (basolateral). [These volumes are a little more than the least required for STX2-PLUS electrode.]
Here are the steps:
Figure 1: STX2-PLUS Adjustment of Electrode Height. Rotate the front ring clockwise so that the electrode can enter to the maximum depth inside the well.

Figure 2: STX2-PLUS Electrode tip and liquid volume requirements. Make sure the electrode sensing tip (red boxed portions) on both blades stay fully immersed in a conductive liquid, such cell culture media or buffer during measurement. You need to have adequate apical and basolateral volumes to get a stable reading. Since STX2-PLUS stays hung, the increased volume must be used to make sure electrode sensing region fully immersed.
NOTE: You must use more liquid volumes than recommended by the insert manufacturer. Insert manufacturer’s recommended volumes will not keep electrode tip fully immersed.
[As mentioned as an example previously, for Corning-24 well Transwell (e.g., Corning 3470) we recommend using minimum 300 µL on top (apical) and 850 µL on bottom (basolateral). These volumes are a little more than the least required for STX2-PLUS electrode. You can check visually to make sure the apical and basolateral volumes are adequate to keep the electrode tips fully immersed, and then consistently use those volumes.]
Even if the unstable reading or dashes issue is still seen, the electrode most probably need chloriding. The chloriding refers to keeping electrode tips immersed in 3-6% sodium hypochlorite or bleach for 10-15 minutes followed by rinsing with distilled water. It is a part of STX2-PLUS maintenance and a critical maintenance process. Please refer to maintenance instruction below (step 1). **
- Can increasing or changing sample liquid volumes change my resistance values?
You can expect to see a change of raw resistance values. However, you subtract the blank values (blank Transwell with no cells) from the sample values (Transwell with cells). This way, you subtract the blank value with increased volume from samples with increased volume. Thus, any change of resistance contributed by increased volume is omitted. Consistently use the same volumes for all your samples in an experiment.
- Is there an electrode cleaning or maintenance instruction that I can follow?
Below are the steps that can be followed for STX2-PLUS cleaning or maintenance. Make sure you use enough liquid levels during cleaning or maintenance at least up to the red boxed region

1. Before using them, chloride the electrode by keeping the electrode tips immersed in 3-6% sodium hypochlorite (bleach) for 10-15 minutes. Chloriding needs to be done every 3 days when the electrodes are used frequently or after more than a week storage. **
2. Rinse with sterile DI water/buffer.
3. Optional step: Quick dip in 70% ethanol or isopropanol and quick dip in DI water/buffer.
4. Use the electrode for measurements.
5. Optional step in between measuring samples: Quick dip in 70% ethanol or isopropanol and quick dip in DI water/buffer.
6. After measurements soak/immerse electrode tips in 70% isopropanol or ethanol for 5-10 minutes.
7. Rinse with DI water. Let it air dry. Store electrode dry and in a place away from light/minimal light.
8. When used frequently, every week soak electrode tips in 1% Tergazyme for 15 minutes. Follow by rinse with DI water.
9. Next, chloride by keeping the electrode tips immersed in 3-6% sodium hypochlorite (bleach) for 10-15 minutes. (Same as step #1.)
10. Rinse with sterile DI water/buffer.
11. Use for measurements.
12. Repeat from step 5.
- Are there any other electrode handing instruction that WPI recommends?
 |
Do NOT hold the electrode by the cable. It can physically break the internal connections gradually. |
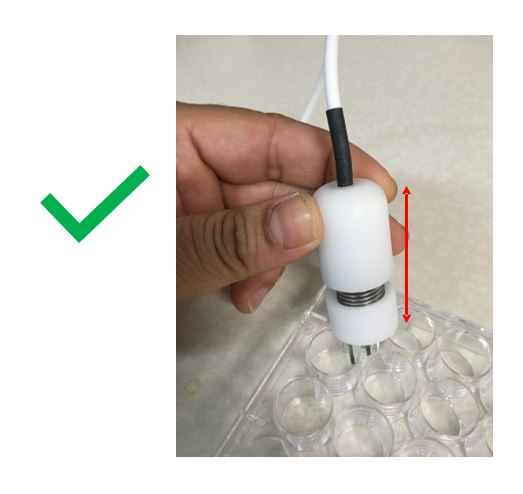 |
Hold the electrode by the arrowed region (plastic). |
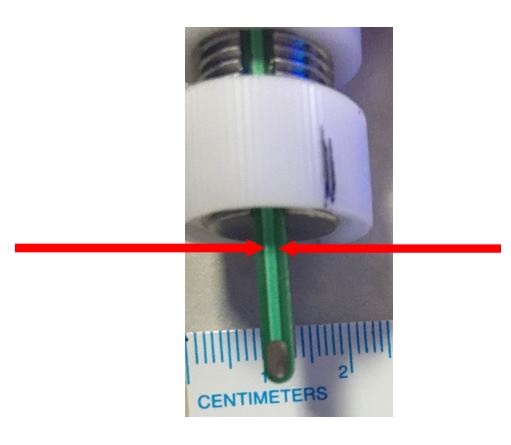 |
Limit liquid immersion or liquid spray level somewhere up to here (maximum). You do not want the liquid to get inside and reach up to internal the cables or connectors that’s why. You can wipe with the rest of the electrode with a paper towel sprayed with isopropanol or ethanol (do not spray directly). |
This unit conforms to the following specifications:
| Type | Descriptions |
| Tissue Sampling Frequency | 12.5 Hz |
| Sample Averaging | 20 samples per second |
| Resistance Ranges |
|
| Auto Mode | 1 to 100,000 Ω auto current 2 μA, 4 μA, 10 μA |
| Resistance Resolution | 0.1 Ω (under 200 Ω); 1 Ω (over 200 Ω) |
| Resistance Accuracy |
|
| Voltage Resolution | 0.001 V, 0.1 mV |
| Accuracy Resistance | 0.1 Ω (200 Ω); 1 Ω (above 200 Ω) |
| Accuracy voltage | ± 0.1 mV |
| Current Levels |
|
| Display Update Rate | 0.5 seconds |
| Battery | 3.7V Li-ion 2500 mAh** |
| Charging Period | 5.5 hours (power off); 6 hours (run time) |
| Charge Current | 200 mA |
| Power Consumption | ~250 mA |
| Certifications | CE |
** mAH means milliamp hours.
Inserts and Plates Compatible with the STX2-PLUS Electrodes
| Corning | Millipore | Material | Membrane Diameter (mm) | Growth Surface Area (cm²) | Membrane Pore Size (μm) |
| 3470 | 6.5 | 0.33 | 0.4 | ||
| 3472 | PITP01250 | 6.5 | 0.33 | 3.0 | |
| 3413 | PCF Insert | 6.5 | 0.33 | 0.4 | |
| 3415 | PITP 01250 PCF Insert |
6.5 | 0.33 | 3.0 | |
| 3421 | 6.5 | 0.33 | 5.0 | ||
| 3422 | PIEP 01250 PCF Insert |
6.5 | 0.33 | 8.0 | |
| 3495 | PIHT12R48* PET Insert |
6.5 | 0.33 | 0.4 | |
| PIHA012 50 | HA Insert | 6.5 | 0.33 | 0.45 | |
| PICM012 50 | CM Insert | 6.5 | 0.33 | 0.4 | |
| 3496 | PISP12R48* PET Insert |
6.5 | 0.33 | 3.0 | |
| PIRP12R48* PET Insert |
6.5 | 0.33 | 1.0 | ||
| PIMP12R48* PET Insert |
6.5 | 0.33 | 5.0 | ||
| PIEP12R48* PET Insert |
6.5 | 0.33 | 8.0 | ||
| PIXP01250 PCF Insert |
6.5 | 0.33 | 12 | ||
| PIHP01250 | 1.0 | ||||
| PITT01250 | 3.0 |
* Tri-supports
| Nunc | Pore size (μm) | Culture area (cm²) |
| 140620 | 0.4 | 0.47 |
| 140627 | 3.0 | 0.47 |
| 140629 | 8.0 | 0.47 |
| ThinCertTM | Membrane material | Pore size [µm] | Pore density [cm-2] | Optical membrane properties | TC surface treatment/Sterile | Multiwell plates/ThinCertTM per box |
| 662640 | PET | 0.4 | 1 x 108 | translucent | +/+ | 2/48 |
| 662641 | PET | 0.4 | 2 x 106 | transparent | +/+ | 2/48 |
| 662610 | PET | 1.0 | 2 x 106 | transparent | +/+ | 2/48 |
| 662630 | PET | 3.0 | 0.6 x 106 | transparent | +/+ | 2/48 |
| 662631 | PET | 3.0 | 2 x 106 | translucent | +/+ | 2/48 |
| 662638 | PET | 8.0 | 0.15 x 106 | translucent | +/+ | 2/48 |
| Millicell | Pore size (μm) | Qty/pk |
| MCHT24H48 | 0.4 | 48 |
| MCRP24H48 | 1.0 | 48 |
| MCSP24H48 | 3.0 | 48 |
| MCMP24H48 | 5.0 | 48 |
| MCEP24H48 | 8.0 | 48 |
| BD Falcon | Membrane material | Pore size [µm] | Pore density [cm-2] | Optical membrane properties | TC plate (#wells) |
| 353095 | PET | 0.4 | 2.0 ± 0.2 x 106 | transparent | 24 |
| 353104 | PET | 1.0 | 1.6 ± 0.6 x 106 | transparent | 24 |
| 353096 | PET | 3.0 | 8 ± 2 x 105 | transparent | 24 |
| 353097 | PET | 8.0 | 6 ± 2 x 104 | translucent | 24 |
| 353495 | PET | 0.4HD | 100 ± 10 x 106 | translucent | 24 |
| 353492 | PET | 3.0HD | 2.0 ± 0.2 x 105 | translucent | 24 |
Shaban, M. S., Müller, C., Mayr-Buro, C., Weiser, H., Meier-Soelch, J., Albert, B. V., … Kracht, M. (2021). Multi-level inhibition of coronavirus replication by chemical ER stress. Nature Communications 2021 12:1, 12(1), 1–20. https://doi.org/10.1038/s41467-021-25551-1
Robinot, R., Hubert, M., de Melo, G. D., Lazarini, F., Bruel, T., Smith, N., … Chakrabarti, L. A. (2021). SARS-CoV-2 infection induces the dedifferentiation of multiciliated cells and impairs mucociliary clearance. Nature Communications 2021 12:1, 12(1), 1–16. https://doi.org/10.1038/s41467-021-24521-x
Pongkorpsakol, P., Turner, J. R., & Zuo, L. (2020). Culture of Intestinal Epithelial Cell Monolayers and Their Use in Multiplex Macromolecular Permeability Assays for In Vitro Analysis of Tight Junction Size Selectivity. Current Protocols in Immunology, 131(1). https://doi.org/10.1002/cpim.112
Neal, E. H., Marinelli, N. A., Shi, Y., McClatchey, P. M., Balotin, K. M., Gullett, D. R., … Lippmann, E. S. (2019). A Simplified, Fully Defined Differentiation Scheme for Producing Blood-Brain Barrier Endothelial Cells from Human iPSCs. Stem Cell Reports, 12(6), 1380–1388. https://doi.org/10.1016/J.STEMCR.2019.05.008
Hollmann, E. K., Bailey, A. K., Potharazu, A. V., Neely, M. D., Bowman, A. B., & Lippmann, E. S. (2017). Accelerated differentiation of human induced pluripotent stem cells to blood–brain barrier endothelial cells. Fluids and Barriers of the CNS 2017 14:1, 14(1), 1–13. https://doi.org/10.1186/S12987-017-0059-0
Stanifer, M. L., Rippert, A., Kazakov, A., Willemsen, J., Bucher, D., Bender, S., … Boulant, S. (2016). Reovirus intermediate subviral particles constitute a strategy to infect intestinal epithelial cells by exploiting TGF-β dependent pro-survival signaling. Cellular Microbiology, 18(12), 1831–1845. https://doi.org/10.1111/cmi.12626
Meenach, S. A., Tsoras, A. N., McGarry, R. C., Mansour, H. M., Hilt, J. Z., & Anderson, K. W. (2016). Development of three-dimensional lung multicellular spheroids in air- and liquid-interface culture for the evaluation of anticancer therapeutics. International Journal of Oncology, 48(4), 1701–1709. https://doi.org/10.3892/ijo.2016.3376
Ferguson, M. C., Saul, S., Fragkoudis, R., Weisheit, S., Cox, J., Patabendige, A., … Fazakerley, J. K. (2015). Ability of the Encephalitic Arbovirus Semliki Forest Virus To Cross the Blood-Brain Barrier Is Determined by the Charge of the E2 Glycoprotein. Journal of Virology, 89(15), 7536–7549. https://doi.org/10.1128/jvi.03645-14
Hollmann, E. K., Bailey, A. K., Potharazu, A. V., Neely, M. D., Bowman, A. B., & Lippmann, E. S. (2017). Accelerated differentiation of human induced pluripotent stem cells to blood–brain barrier endothelial cells. Fluids and Barriers of the CNS 2017 14:1, 14(1), 1–13. https://doi.org/10.1186/S12987-017-0059-0
Neal, E. H., Marinelli, N. A., Shi, Y., McClatchey, P. M., Balotin, K. M., Gullett, D. R., … Lippmann, E. S. (2019). A Simplified, Fully Defined Differentiation Scheme for Producing Blood-Brain Barrier Endothelial Cells from Human iPSCs. Stem Cell Reports, 12(6), 1380–1388. https://doi.org/10.1016/J.STEMCR.2019.05.008
Erami, Z., Timpson, P., Yao, W., Zaidel-Bar, R., & Anderson, K. I. (2015). There are four dynamically and functionally distinct populations of E-cadherin in cell junctions. Biology Open, 4(11), 1481–1489. https://doi.org/10.1242/bio.014159
Tosoni, K., Cassidy, D., Kerr, B., Land, S. C., & Mehta, A. (2016). Using Drugs to Probe the Variability of Trans-Epithelial Airway Resistance. PloS One, 11(2), e0149550. https://doi.org/10.1371/journal.pone.0149550
Venter, J., Francis, H., Meng, F., DeMorrow, S., Kennedy, L., Standeford, H., … Alpini, G. (2015). Development and functional characterization of extrahepatic cholangiocyte lines from normal rats. Digestive and Liver Disease : Official Journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver, 47(11), 964–972. https://doi.org/10.1016/j.dld.2015.07.012
Noh, S. Y., Kang, S.-S., Yun, C.-H., & Han, S. H. (2015). Lipoteichoic acid from Lactobacillus plantarum inhibits Pam2CSK4-induced IL-8 production in human intestinal epithelial cells. Molecular Immunology, 64(1), 183–189. https://doi.org/10.1016/j.molimm.2014.11.014
Wang, Y., & Alexander, J. S. (2011). Analysis of endothelial barrier function in vitro. Methods in Molecular Biology, 763, 253–264. https://doi.org/10.1007/978-1-61779-191-8_17
Ghaffarian, R., & Muro, S. (2013). Models and Methods to Evaluate Transport of Drug Delivery Systems Across Cellular Barriers. Journal of Visualized Experiments, (80), e50638–e50638. https://doi.org/10.3791/50638
Dickman, K. G., Hempson, S. J., Anderson, J., Lippe, S., Zhao, L., Burakoff, R., & Shaw, R. D. (n.d.). Rotavirus alters paracellular permeability and energy metabolism in Caco-2 cells.
Pham, V. T., Seifert, N., Richard, N., Raederstorff, D., Steinert, R., Prudence, K., & Mohajeri, M. H. (2018). The effects of fermentation products of prebiotic fibres on gut barrier and immune functions in vitro. PeerJ, 6, e5288. https://doi.org/10.7717/peerj.5288
Bu, HZ; Poglod, M; Micetich, RG; Khan, J. (2000). High-throughput Caco-2 cell permeability screening by cassette dosing and sample pooling approaches using direct injection/on-line guard cartridge extraction/tandem mass spectrometry. Retrieved November 15, 2016, from http://serials.unibo.it/cgi-ser/start/en/spogli/df-s.tcl?prog_art=7030068&language=ENGLISH&view=articoli
Roos, S., Wyder, M., Candi, A., Regenscheit, N., Nathues, C., van Immerseel, F., & Posthaus, H. (2015). Binding studies on isolated porcine small intestinal mucosa and in vitro toxicity studies reveal lack of effect of C. perfringens beta-toxin on the porcine intestinal epithelium. Toxins, 7(4), 1235–1252. https://doi.org/10.3390/toxins7041235
Shang, V. C. M., Kendall, D. A., & Roberts, R. E. (2016). Δ9-Tetrahydrocannabinol reverses TNFα-induced increase in airway epithelial cell permeability through CB2 receptors. Biochemical Pharmacology, 120, 63–71. https://doi.org/10.1016/j.bcp.2016.09.008
Turdalieva, A., Solandt, J., Shambetova, N., Xu, H., Blom, H., Brismar, H., … Fu, Y. (2016). Bioelectric and Morphological Response of Liquid-Covered Human Airway Epithelial Calu-3 Cell Monolayer to Periodic Deposition of Colloidal 3-Mercaptopropionic-Acid Coated CdSe-CdS/ZnS Core-Multishell Quantum Dots. PloS One, 11(2), e0149915. https://doi.org/10.1371/journal.pone.0149915
Utsumi, H., Chiba, H., Kamimura, Y., Osanai, M., Igarashi, Y., Tobioka, H., … Sawada, N. (2000). Expression of GFRα-1, receptor for GDNF, in rat brain capillary during postnatal development of the BBB. American Journal of Physiology - Cell Physiology, 279(2).
Czupalla, C. J., Liebner, S., & Devraj, K. (2014). In vitro models of the blood-brain barrier. Methods in Molecular Biology (Clifton, N.J.), 1135, 415–437. https://doi.org/10.1007/978-1-4939-0320-7_34
Li, Y., Wu, W.-H., Hsu, C.-W., Nguyen, H. V, Tsai, Y.-T., Chan, L., … Tsang, S. H. (2014). Gene therapy in patient-specific stem cell lines and a preclinical model of retinitis pigmentosa with membrane frizzled-related protein defects. Molecular Therapy : The Journal of the American Society of Gene Therapy, 22(9), 1688–1697. https://doi.org/10.1038/mt.2014.100
Xu, Q., Liu, J., Wang, Z., Guo, X., Zhou, G., Liu, Y., … Su, L. (2015). Heat stress-induced disruption of endothelial barrier function is via PAR1 signaling and suppressed by Xuebijing injection. PloS One, 10(2), e0118057. https://doi.org/10.1371/journal.pone.0118057
Reichert, M., Müller, T., & Hunziker, W. (2000). The PDZ domains of zonula occludens-1 induce an epithelial to mesenchymal transition of Madin-Darby canine kidney I cells. Evidence for a role of beta-catenin/Tcf/Lef signaling. The Journal of Biological Chemistry, 275(13), 9492–9500. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10734097
Laksitorini, M. D., Kiptoo, P. K., On, N. H., Thliveris, J. A., Miller, D. W., & Siahaan, T. J. (2015). Modulation of intercellular junctions by cyclic-ADT peptides as a method to reversibly increase blood-brain barrier permeability. Journal of Pharmaceutical Sciences, 104(3), 1065–1075. https://doi.org/10.1002/jps.24309
Zhang, J., Field, C. J., Vine, D., & Chen, L. (2015). Intestinal Uptake and Transport of Vitamin B12-loaded Soy Protein Nanoparticles. Pharmaceutical Research, 32(4), 1288–1303. https://doi.org/10.1007/s11095-014-1533-x
Lippmann, E. S., Azarin, S. M., Kay, J. E., Nessler, R. A., Wilson, H. K., Al-Ahmad, A., … Shusta, E. V. (2012). Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nature Biotechnology, 30(8), 783–791. https://doi.org/10.1038/nbt.2247
Alhamoruni, A., Lee, A. C., Wright, K. L., Larvin, M., & O’Sullivan, S. E. (2010). Pharmacological Effects of Cannabinoids on the Caco-2 Cell Culture Model of Intestinal Permeability. Journal of Pharmacology and Experimental Therapeutics, 335(1).
Kauffman, A. L., Gyurdieva, A. V., Mabus, J. R., Ferguson, C., Yan, Z., & Hornby, P. J. (2013). Alternative functional in vitro models of human intestinal epithelia. Frontiers in Pharmacology, 4, 79. https://doi.org/10.3389/fphar.2013.00079
Chen, L., Zhu, J., Li, Y., Lu, J., Gao, L., Xu, H., … Yang, X. (2013). Enhanced nasal mucosal delivery and immunogenicity of anti-caries DNA vaccine through incorporation of anionic liposomes in chitosan/DNA complexes. PloS One, 8(8), e71953. https://doi.org/10.1371/journal.pone.0071953
Meenach, S. A., Tsoras, A. N., McGarry, R. C., Mansour, H. M., Hilt, J. Z., & Anderson, K. W. (2016). Development of three-dimensional lung multicellular spheroids in air- and liquid-interface culture for the evaluation of anticancer therapeutics. International Journal of Oncology, 48(4), 1701–1709. https://doi.org/10.3892/ijo.2016.3376
Reaves, D. K., Fagan-Solis, K. D., Dunphy, K., Oliver, S. D., Scott, D. W., & Fleming, J. M. (2014). The role of lipolysis stimulated lipoprotein receptor in breast cancer and directing breast cancer cell behavior. PloS One, 9(3), e91747. https://doi.org/10.1371/journal.pone.0091747
Brown, J. A., Pensabene, V., Markov, D. A., Allwardt, V., Neely, M. D., Shi, M., … Wikswo, J. P. (2015). Recreating blood-brain barrier physiology and structure on chip: A novel neurovascular microfluidic bioreactor. Biomicrofluidics, 9(5), 054124. https://doi.org/10.1063/1.4934713
Lei, Y., Stamer, W. D., Wu, J., & Sun, X. (2014). Cell senescence reduced the mechanotransduction sensitivity of porcine angular aqueous plexus cells to elevation of pressure. Investigative Ophthalmology & Visual Science, 55(4), 2324–2328. https://doi.org/10.1167/iovs.13-13317
Torr, E., Heath, M., Mee, M., Shaw, D., Sharp, T. V, & Sayers, I. (2016). Expression of polycomb protein BMI-1 maintains the plasticity of basal bronchial epithelial cells. Physiological Reports, 4(16). https://doi.org/10.14814/phy2.12847
Lei, Y., Stamer, W. D., Wu, J., & Sun, X. (2013). Oxidative stress impact on barrier function of porcine angular aqueous plexus cell monolayers. Investigative Ophthalmology & Visual Science, 54(7), 4827–4835. https://doi.org/10.1167/iovs.12-11435
Sheller, R. A., Cuevas, M. E., & Todd, M. C. (2017). Comparison of transepithelial resistance measurement techniques: Chopsticks vs. Endohm. Biological Procedures Online, 19, 4. https://doi.org/10.1186/s12575-017-0053-6
Melvin, J. A., Lashua, L. P., Kiedrowski, M. R., Yang, G., Deslouches, B., Montelaro, R. C., & Bomberger, J. M. (2016). Simultaneous Antibiofilm and Antiviral Activities of an Engineered Antimicrobial Peptide during Virus-Bacterium Coinfection. MSphere, 1(3). https://doi.org/10.1128/mSphere.00083-16
Campbell, L., Abulrob, A.-N. G., Kandalaft, L. E., Plummer, S., Hollins, A. J., Gibbs, A., & Gumbleton, M. (2003). Constitutive Expression of P-Glycoprotein in Normal Lung Alveolar Epithelium and Functionality in Primary Alveolar Epithelial Cultures. Journal of Pharmacology and Experimental Therapeutics, 304(1).
Richter, J. F., Schmauder, R., Krug, S. M., Gebert, A., & Schumann, M. (2016). A novel method for imaging sites of paracellular passage of macromolecules in epithelial sheets. Journal of Controlled Release, 229, 70–79. https://doi.org/10.1016/j.jconrel.2016.03.018
Meenach, S. A., Anderson, K. W., Hilt, J. Z., McGarry, R. C., Mansour, H. M., Samantha A. Meenach, Kimberly W. Anderson, J. Zach Hilt, Ronald C. McGarry, H. M. M., … Mansour, H. M. (2014, December 20). High-Performing Dry Powder Inhalers of Paclitaxel DPPC/DPPG Lung Surfactant-Mimic Multifunctional Particles in Lung Cancer: Physicochemical Characterization, In Vitro Aerosol Dispersion, and Cellular Studies. https://doi.org/10.1208/s12249-014-0182-z
Schexnayder, C., & Stratford, R. E. (2015). Genistein and Glyceollin Effects on ABCC2 (MRP2) and ABCG2 (BCRP) in Caco-2 Cells. International Journal of Environmental Research and Public Health, 13(1), ijerph13010017. https://doi.org/10.3390/ijerph13010017
Pongkorpsakol, P., Turner, J. R., & Zuo, L. (2020). Culture of Intestinal Epithelial Cell Monolayers and Their Use in Multiplex Macromolecular Permeability Assays for In Vitro Analysis of Tight Junction Size Selectivity. Current Protocols in Immunology, 131(1). https://doi.org/10.1002/cpim.112
Grover, A., Hirani, A., Pathak, Y., & Sutariya, V. (2014). Brain-targeted delivery of docetaxel by glutathione-coated nanoparticles for brain cancer. AAPS PharmSciTech, 15(6), 1562–1568. https://doi.org/10.1208/s12249-014-0165-0
Meerveld, G.-V. B., R, T. K., 文 タ イ ト ル和, & vitro の ラ ッ ト 空 腸 お よ び 結 腸 に お け る 木 ク レ オ ソ - ト お よ び ロ ペ ラ ミ ド の 止 瀉 効 果 の 比 較, I. (2000). Comparison of the antidiarrheal effects of wood creosote and loperamide in the rat jejunum and colon in vitro. Biol Pharm Bull, 23, 952–956.
Pongkorpsakol, P., Pathomthongtaweechai, N., Srimanote, P., Soodvilai, S., Chatsudthipong, V., & Muanprasat, C. (2014). Inhibition of cAMP-activated intestinal chloride secretion by diclofenac: cellular mechanism and potential application in cholera. PLoS Neglected Tropical Diseases, 8(9), e3119. https://doi.org/10.1371/journal.pntd.0003119
Li, G., Li, T., Li, Y., Cai, S., Zhang, Z., Zeng, Z., … Chen, Z. (2014). Ulinastatin inhibits oxidant-induced endothelial hyperpermeability and apoptotic signaling. International Journal of Clinical and Experimental Pathology, 7(11), 7342–7350. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/25550770
Wise, S. K., Laury, A. M., Katz, E. H., Den Beste, K. A., Parkos, C. A., & Nusrat, A. (2014). Interleukin-4 and interleukin-13 compromise the sinonasal epithelial barrier and perturb intercellular junction protein expression. International Forum of Allergy & Rhinology, 4(5), 361–370. https://doi.org/10.1002/alr.21298
Blenkinsop, T. A., Saini, J. S., Maminishkis, A., Bharti, K., Wan, Q., Banzon, T., … Stern, J. H. (2015). Human Adult Retinal Pigment Epithelial Stem Cell-Derived RPE Monolayers Exhibit Key Physiological Characteristics of Native Tissue. Investigative Ophthalmology & Visual Science, 56(12), 7085–7099. https://doi.org/10.1167/iovs.14-16246
Park, S. W., Kim, J. H., Park, S. M., Moon, M., Lee, K. H., Park, K. H., … Kim, J. H. (2015). RAGE mediated intracellular Aβ uptake contributes to the breakdown of tight junction in retinal pigment epithelium. Oncotarget, 6(34), 35263–35273. https://doi.org/10.18632/oncotarget.5894
Saeedi, B. J., Kao, D. J., Kitzenberg, D. A., Dobrinskikh, E., Schwisow, K. D., Masterson, J. C., … Glover, L. E. (2015). HIF-dependent regulation of claudin-1 is central to intestinal epithelial tight junction integrity. Molecular Biology of the Cell, 26(12), 2252–2262. https://doi.org/10.1091/mbc.E14-07-1194
Fossum, S. L., Mutolo, M. J., Yang, R., Dang, H., O’Neal, W. K., Knowles, M. R., … Harris, A. (2014). Ets homologous factor regulates pathways controlling response to injury in airway epithelial cells. Nucleic Acids Research, 42(22), 13588–13598. https://doi.org/10.1093/nar/gku1146
Cao, X., Lin, H., Muskhelishvili, L., Latendresse, J., Richter, P., Heflich, R. H., … Browning, M. (2015). Tight junction disruption by cadmium in an in vitro human airway tissue model, 16(1), 30. https://doi.org/10.1186/s12931-015-0191-9
Booth, R., & Kim, H. (2014). Permeability Analysis of Neuroactive Drugs Through a Dynamic Microfluidic In Vitro Blood–Brain Barrier Model. Annals of Biomedical Engineering, 42(12), 2379–2391. https://doi.org/10.1007/s10439-014-1086-5
Mooren, O. L., Kim, J., Li, J., & Cooper, J. A. (2015). Role of N-WASP in Endothelial Monolayer Formation and Integrity. Journal of Biological Chemistry, 290(30), 18796–18805. https://doi.org/10.1074/jbc.M115.668285
Bernocchi, B., Carpentier, R., Lantier, I., Ducournau, C., Dimier-Poisson, I., & Betbeder, D. (2016). Mechanisms allowing protein delivery in nasal mucosa using NPL nanoparticles. Journal of Controlled Release : Official Journal of the Controlled Release Society, 232, 42–50. https://doi.org/10.1016/j.jconrel.2016.04.014
Byeon, H. J., Thao, L. Q., Lee, S., Min, S. Y., Lee, E. S., Shin, B. S., … Youn, Y. S. (2016). Doxorubicin-loaded nanoparticles consisted of cationic- and mannose-modified-albumins for dual-targeting in brain tumors. Journal of Controlled Release, 225, 301–313. https://doi.org/10.1016/j.jconrel.2016.01.046
Crane, J. K., Broome, J. E., Reddinger, R. M., & Werth, B. B. (2014). Zinc protects against Shiga-toxigenic Escherichia coli by acting on host tissues as well as on bacteria. BMC Microbiology, 14, 145. https://doi.org/10.1186/1471-2180-14-145
Ahmed, C. M., Biswal, M. R., Li, H., Han, P., Ildefonso, C. J., & Lewin, A. S. (2016). Repurposing an orally available drug for the treatment of geographic atrophy. Molecular Vision, 22, 294–310. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/27110092
Cieza, R. J., Hu, J., Ross, B. N., Sbrana, E., & Torres, A. G. (2015). The IbeA invasin of adherent-invasive Escherichia coli mediates interaction with intestinal epithelia and macrophages. Infection and Immunity, 83(5), 1904–1918. https://doi.org/10.1128/IAI.03003-14
Leir, S.-H., Browne, J. A., Eggener, S. E., & Harris, A. (2015). Characterization of primary cultures of adult human epididymis epithelial cells. Fertility and Sterility, 103(3), 647-54.e1. https://doi.org/10.1016/j.fertnstert.2014.11.022
Wise, S. K., Laury, A. M., Katz, E. H., Den Beste, K. A., Parkos, C. A., & Nusrat, A. (2014). Interleukin-4 and interleukin-13 compromise the sinonasal epithelial barrier and perturb intercellular junction protein expression. International Forum of Allergy & Rhinology, 4(5), 361–370. https://doi.org/10.1002/alr.21298
Ferguson, M. C., Saul, S., Fragkoudis, R., Weisheit, S., Cox, J., Patabendige, A., … Fazakerley, J. K. (2015). Ability of the Encephalitic Arbovirus Semliki Forest Virus To Cross the Blood-Brain Barrier Is Determined by the Charge of the E2 Glycoprotein. Journal of Virology, 89(15), 7536–7549. https://doi.org/10.1128/JVI.03645-14
Ross, B. N., Rojas-Lopez, M., Cieza, R. J., McWilliams, B. D., & Torres, A. G. (2015). The Role of Long Polar Fimbriae in Escherichia coli O104:H4 Adhesion and Colonization. PLOS ONE, 10(10), e0141845. https://doi.org/10.1371/journal.pone.0141845
Kuehn, D., Majeed, S., Guedj, E., Dulize, R., Baumer, K., Iskandar, A., … Peitsch, M. C. (2015). Impact Assessment of Repeated Exposure of Organotypic 3D Bronchial and Nasal Tissue Culture Models to Whole Cigarette Smoke. Journal of Visualized Experiments, (96), e52325–e52325. https://doi.org/10.3791/52325
Castro, R., Barlow-Walden, L., Woodson, T., Kerecman, J. D., Zhang, G. H., & Martinez, J. R. (2000). Ion transport in an immortalized rat submandibular cell line SMG-C6. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, N.Y.), 225(1), 39–48. https://doi.org/10.1046/J.1525-1373.2000.22505.X
Pastor-Clerigues, A., Serrano, A., Milara, J., Marti-Bonmati, E., Lopez-Perez, F. J., Garcia-Montanes, S., … Cortijo, J. (2016). Evaluation of the Ocular Tolerance of Three Tacrolimus Topical Pharmaceutical Preparations by Bovine Corneal Opacity and Permeability Test. Current Eye Research, 41(7), 890–896. https://doi.org/10.3109/02713683.2015.1082187
Mishra, R., & Singh, S. K. (2014). HIV-1 Tat C phosphorylates VE-cadherin complex and increases human brain microvascular endothelial cell permeability. BMC Neuroscience, 15, 80. https://doi.org/10.1186/1471-2202-15-80
Guzman-Aranguez, A., Calvo, P., Ropero, I., & Pintor, J. (2014). In vitro effects of preserved and unpreserved anti-allergic drugs on human corneal epithelial cells. Journal of Ocular Pharmacology and Therapeutics : The Official Journal of the Association for Ocular Pharmacology and Therapeutics, 30(9), 790–798. https://doi.org/10.1089/jop.2014.0030
Odijk, M., van der Meer, A. D., Levner, D., Kim, H. J., van der Helm, M. W., Segerink, L. I., … van den Berg, A. (2015). Measuring direct current trans-epithelial electrical resistance in organ-on-a-chip microsystems. Lab on a Chip, 15(3), 745–752. https://doi.org/10.1039/c4lc01219d
Mansourpour, M., Mahjub, R., Amini, M., Ostad, S. N., Shamsa, E. S., Rafiee-Tehrani, M., & Dorkoosh, F. A. (2015). Development of acid-resistant alginate/trimethyl chitosan nanoparticles containing cationic β-cyclodextrin polymers for insulin oral delivery. AAPS PharmSciTech, 16(4), 952–962. https://doi.org/10.1208/s12249-014-0282-9
Sjöqvist, S., Jungebluth, P., Lim, M. L., Haag, J. C., Gustafsson, Y., Lemon, G., … Macchiarini, P. (2014). Experimental orthotopic transplantation of a tissue-engineered oesophagus in rats. Nature Communications, 5, 3562. https://doi.org/10.1038/ncomms4562
Roh-Johnson, M., Bravo-Cordero, J. J., Patsialou, A., Sharma, V. P., Guo, P., Liu, H., … Condeelis, J. (2014). Macrophage contact induces RhoA GTPase signaling to trigger tumor cell intravasation. Oncogene, 33(33), 4203–4212. https://doi.org/10.1038/onc.2013.377
Senyavina, N. V., & Tonevitskaya, S. A. (2015). Effect of Hypoxanthine on Functional Activity of Nucleoside Transporters ENT1 and ENT2 in Caco-2 Polar Epithelial Intestinal Cells. Bulletin of Experimental Biology and Medicine, 160(1), 160–164. https://doi.org/10.1007/s10517-015-3118-z
Khan, N., Pantakani, D. V. K., Binder, L., Qasim, M., & Asif, A. R. (2015). Immunosuppressant MPA Modulates Tight Junction through Epigenetic Activation of MLCK/MLC-2 Pathway via p38MAPK. Frontiers in Physiology, 6, 381. https://doi.org/10.3389/fphys.2015.00381
Couturier, J., Hutchison, A. T., Medina, M. A., Gingaras, C., Urvil, P., Yu, X., … Lewis, D. E. (2014). HIV replication in conjunction with granzyme B production by CCR5+ memory CD4 T cells: Implications for bystander cell and tissue pathologies. Virology, 462–463, 175–188. https://doi.org/10.1016/j.virol.2014.06.008
Biswal, M. R., Ahmed, C. M., Ildefonso, C. J., Han, P., Li, H., Jivanji, H., … Lewin, A. S. (2015). Systemic treatment with a 5HT1a agonist induces anti-oxidant protection and preserves the retina from mitochondrial oxidative stress. Experimental Eye Research, 140, 94–105. https://doi.org/10.1016/j.exer.2015.07.022
Netsomboon, K., Laffleur, F., & Bernkop-Schnürch, A. (2016). P-glycoprotein inhibitors: synthesis and in vitro evaluation of a preactivated thiomer. Drug Development and Industrial Pharmacy, 42(4), 668–675. https://doi.org/10.3109/03639045.2015.1075025
Lewis, S. B., Cook, V., Tighe, R., & Schüller, S. (2015). Enterohemorrhagic Escherichia coli colonization of human colonic epithelium in vitro and ex vivo. Infection and Immunity, 83(3), 942–949. https://doi.org/10.1128/IAI.02928-14
Yan, Y., Shapiro, A. P., Mopidevi, B. R., Chaudhry, M. A., Maxwell, K., Haller, S. T., … Liu, J. (2016). Protein Carbonylation of an Amino Acid Residue of the Na/K-ATPase α1 Subunit Determines Na/K-ATPase Signaling and Sodium Transport in Renal Proximal Tubular Cells. Journal of the American Heart Association, 5(9). https://doi.org/10.1161/JAHA.116.003675
Wang, G., Zabner, J., Deering, C., Launspach, J., Shao, J., Bodner, M., … McCray, P. B. (2000). Increasing Epithelial Junction Permeability Enhances Gene Transfer to Airway Epithelia In Vivo. American Journal of Respiratory Cell and Molecular Biology, 22(2), 129–138. https://doi.org/10.1165/ajrcmb.22.2.3938
Zaccone, E. J., Goldsmith, W. T., Shimko, M. J., Wells, J. R., Schwegler-Berry, D., Willard, P. A., … Fedan, J. S. (2015). Diacetyl and 2,3-pentanedione exposure of human cultured airway epithelial cells: Ion transport effects and metabolism of butter flavoring agents. Toxicology and Applied Pharmacology, 289(3), 542–549. https://doi.org/10.1016/j.taap.2015.10.004
Czupalla, C. J., Liebner, S., & Devraj, K. (2014). In Vitro Models of the Blood–Brain Barrier (pp. 415–437). https://doi.org/10.1007/978-1-4939-0320-7_34
Sei, Y. J., Ahn, S. I., Virtue, T., Kim, T., & Kim, Y. (2017). Detection of frequency-dependent endothelial response to oscillatory shear stress using a microfluidic transcellular monitor. Scientific Reports, 7(1), 10019. https://doi.org/10.1038/s41598-017-10636-z
Inglis, V. I., Jones, M. P. J., Tse, A. D. Y., & Easton, A. S. (2004). Neutrophils both reduce and increase permeability in a cell culture model of the blood–brain barrier. Brain Research, 998(2), 218–229. https://doi.org/10.1016/J.BRAINRES.2003.11.031
Xing, F., Sharma, S., Liu, Y., Mo, Y.-Y., Wu, K., Zhang, Y.-Y., … Watabe, K. (2015). miR-509 suppresses brain metastasis of breast cancer cells by modulating RhoC and TNF-α. Oncogene, 34(37), 4890–4900. https://doi.org/10.1038/onc.2014.412
Booth, R., & Kim, H. (2012). Characterization of a microfluidic in vitro model of the blood-brain barrier (μBBB). Lab on a Chip, 12(10), 1784. https://doi.org/10.1039/c2lc40094d
Srinivasan, B., Kolli, A. R., Esch, M. B., Abaci, H. E., Shuler, M. L., & Hickman, J. J. (2015). TEER measurement techniques for in vitro barrier model systems. Journal of Laboratory Automation, 20(2), 107–126. https://doi.org/10.1177/2211068214561025
S. Jayaraman, Y. Song, L.Vetrivel, L.Shankar, A. S. V. (2001). Noninvasive in vivo fluorescence measurement of airway-surface liquid depth, salt concentration, and pH. The Journal of Clinical Investigation, 107(3), 317. https://doi.org/10.1172/JCI11154
Chen, S., Einspanier, R., & Schoen, J. (2015). Transepithelial electrical resistance (TEER): a functional parameter to monitor the quality of oviduct epithelial cells cultured on filter supports. Histochemistry and Cell Biology, 144(5), 509–515. https://doi.org/10.1007/s00418-015-1351-1
Yu, J., Li, N., Lin, P., Li, Y., Mao, X., Bao, G., … Zhao, R. (2014). Intestinal transportations of main chemical compositions of polygoni multiflori radix in caco-2 cell model. Evidence-Based Complementary and Alternative Medicine : ECAM, 2014, 483641. https://doi.org/10.1155/2014/483641
Pothoven, K. L., Norton, J. E., Hulse, K. E., Suh, L. A., Carter, R. G., Rocci, E., … Schleimer, R. P. (2015). Oncostatin M promotes mucosal epithelial barrier dysfunction, and its expression is increased in patients with eosinophilic mucosal disease. The Journal of Allergy and Clinical Immunology, 136(3), 737-746.e4. https://doi.org/10.1016/j.jaci.2015.01.043
Williams, K. M., Gokulan, K., Cerniglia, C. E., & Khare, S. (2016). Size and dose dependent effects of silver nanoparticle exposure on intestinal permeability in an in vitro model of the human gut epithelium. Journal of Nanobiotechnology, 14(1), 62. https://doi.org/10.1186/s12951-016-0214-9
Johnson, L. G., Olsen, J. C., Naldini, L., & Boucher, R. C. (2000). Pseudotyped human lentiviral vector-mediated gene transfer to airway epithelia in vivo. Gene Therapy, 7(7), 568–574. https://doi.org/10.1038/sj.gt.3301138
Li, Q., Chen, B., Zeng, C., Fan, A., Yuan, Y., Guo, X., … Huang, Q. (2015). Differential activation of receptors and signal pathways upon stimulation by different doses of sphingosine-1-phosphate in endothelial cells. Experimental Physiology, 100(1), 95–107. https://doi.org/10.1113/expphysiol.2014.082149
Busch, C., Hofmann, F., Gerhard, R., & Aktories, K. (2000). Involvement of a conserved tryptophan residue in the UDP-glucose binding of large clostridial cytotoxin glycosyltransferases. The Journal of Biological Chemistry, 275(18), 13228–13234. https://doi.org/10.1074/JBC.275.18.13228
Healy, L. L., Cronin, J. G., & Sheldon, I. M. (2015). Polarized Epithelial Cells Secrete Interleukin 6 Apically in the Bovine Endometrium1. Biology of Reproduction, 92(6), 151. https://doi.org/10.1095/biolreprod.115.127936
Enjoji, S., Ohama, T., & Sato, K. (2014). Regulation of epithelial cell tight junctions by protease-activated receptor 2. The Journal of Veterinary Medical Science, 76(9), 1225–1229. https://doi.org/10.1292/jvms.14-0191
Maherally, Z., Fillmore, H. L., Tan, S. L., Tan, S. F., Jassam, S. A., Quack, F. I., … Pilkington, G. J. (2018). Real‐time acquisition of transendothelial electrical resistance in an all‐human, in vitro , 3‐dimensional, blood‐brain barrier model exemplifies tight‐junction integrity. The FASEB Journal, 32(1), 168–182. https://doi.org/10.1096/fj.201700162R
Blaisdell, C. J., Edmonds, R. D., Wang, X. T., Guggino, S., & Zeitlin, P. L. (2000). pH-regulated chloride secretion in fetal lung epithelia. American Journal of Physiology. Lung Cellular and Molecular Physiology, 278(6), L1248-55. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10835331
Di, S., Gujie, M., & Thomas, W. (2016). Magnetic ferri-liposomes for triggered drug release across the blood- brain barrier. Frontiers in Bioengineering and Biotechnology, 4. https://doi.org/10.3389/conf.FBIOE.2016.01.00061
Costello, C. M., Hongpeng, J., Shaffiey, S., Yu, J., Jain, N. K., Hackam, D., & March, J. C. (2014). Synthetic small intestinal scaffolds for improved studies of intestinal differentiation. Biotechnology and Bioengineering, 111(6), 1222–1232. https://doi.org/10.1002/bit.25180
Molenda, N., Urbanova, K., Weiser, N., Kusche-Vihrog, K., Günzel, D., Schillers, H., … Howell, S. (2014). Paracellular Transport through Healthy and Cystic Fibrosis Bronchial Epithelial Cell Lines – Do We Have a Proper Model? PLoS ONE, 9(6), e100621. https://doi.org/10.1371/journal.pone.0100621
Costello, C. M., Sorna, R. M., Goh, Y.-L., Cengic, I., Jain, N. K., & March, J. C. (2014). 3-D intestinal scaffolds for evaluating the therapeutic potential of probiotics. Molecular Pharmaceutics, 11(7), 2030–2039. https://doi.org/10.1021/mp5001422
Iacovelli, J., Rowe, G. C., Khadka, A., Diaz-Aguilar, D., Spencer, C., Arany, Z., & Saint-Geniez, M. (2016). PGC-1α Induces Human RPE Oxidative Metabolism and Antioxidant Capacity. Investigative Ophthalmology & Visual Science, 57(3), 1038–1051. https://doi.org/10.1167/iovs.15-17758
Bartakova, A., Alvarez-Delfin, K., Weisman, A. D., Salero, E., Raffa, G. A., Merkhofer, R. M., … Goldberg, J. L. (2016). Novel Identity and Functional Markers for Human Corneal Endothelial Cells. Investigative Ophthalmology & Visual Science, 57(6), 2749–2762. https://doi.org/10.1167/iovs.15-18826
Srimanee, A., Regberg, J., Hällbrink, M., Vajragupta, O., & Langel, Ü. (2016). Role of scavenger receptors in peptide-based delivery of plasmid DNA across a blood–brain barrier model. International Journal of Pharmaceutics, 500(1–2), 128–135. https://doi.org/10.1016/j.ijpharm.2016.01.014
Garate, M. A., & Nunez, M. T. (2000). Overexpression of the Ferritin Iron-responsive Element Decreases the Labile Iron Pool and Abolishes the Regulation of Iron Absorption by Intestinal Epithelial (Caco-2) Cells. Journal of Biological Chemistry, 275(3), 1651–1655. https://doi.org/10.1074/jbc.275.3.1651
Yang, J. J., Kim, K. J., & Lee, V. H. (2000). Role of P-glycoprotein in restricting propranolol transport in cultured rabbit conjunctival epithelial cell layers. Pharmaceutical Research, 17(5), 533–538. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10888304
Zhang, J., Ni, C., Yang, Z., Piontek, A., Chen, H., Wang, S., … Piontek, J. (2015). Specific binding of Clostridium perfringens enterotoxin fragment to Claudin-b and modulation of zebrafish epidermal barrier. Experimental Dermatology, 24(8), 605–610. https://doi.org/10.1111/exd.12728
Lewis, S. B., Prior, A., Ellis, S. J., Cook, V., Chan, S. S. M., Gelson, W., & Schüller, S. (2016). Flagellin Induces β-Defensin 2 in Human Colonic Ex vivo Infection with Enterohemorrhagic Escherichia coli. Frontiers in Cellular and Infection Microbiology, 6, 68. https://doi.org/10.3389/fcimb.2016.00068
Marvin, S. A., Huerta, C. T., Sharp, B., Freiden, P., Cline, T. D., & Schultz-Cherry, S. (2016). Type I Interferon Response Limits Astrovirus Replication and Protects against Increased Barrier Permeability In Vitro and In Vivo. Journal of Virology, 90(4), 1988–1996. https://doi.org/10.1128/JVI.02367-15
Shirasawa, M., Sonoda, S., Terasaki, H., Arimura, N., Otsuka, H., Yamashita, T., … Sakamoto, T. (2013). TNF-α disrupts morphologic and functional barrier properties of polarized retinal pigment epithelium. Experimental Eye Research, 110, 59–69. https://doi.org/10.1016/j.exer.2013.02.012
Prewitt, A. R., Ghose, S., Frump, A. L., Datta, A., Austin, E. D., Kenworthy, A. K., & de Caestecker, M. P. (2015). Heterozygous null bone morphogenetic protein receptor type 2 mutations promote SRC kinase-dependent caveolar trafficking defects and endothelial dysfunction in pulmonary arterial hypertension. The Journal of Biological Chemistry, 290(2), 960–971. https://doi.org/10.1074/jbc.M114.591057
Loma, P., Guzman-Aranguez, A., Pérez de Lara, M. J., & Pintor, J. (2015). Diadenosine tetraphosphate induces tight junction disassembly thus increasing corneal epithelial permeability. British Journal of Pharmacology, 172(4), 1045–1058. https://doi.org/10.1111/bph.12972
De Chiara, L., Fagoonee, S., Ranghino, A., Bruno, S., Camussi, G., Tolosano, E., … Altruda, F. (2014). Renal cells from spermatogonial germline stem cells protect against kidney injury. Journal of the American Society of Nephrology : JASN, 25(2), 316–328. https://doi.org/10.1681/ASN.2013040367
Sawai, T., Usui, N., Dwaihy, J., Drongowski, R. A., Abe, A., Coran, A. G., & Harmon, C. M. (n.d.). The effect of phospholipase A2 on bacterial translocation in a cell culture model. Pediatric Surgery International, 16(4), 262–266. https://doi.org/10.1007/S003830050741
Ao, M., Sarathy, J., Domingue, J., Alrefai, W. A., & Rao, M. C. (2013). Chenodeoxycholic acid stimulates Cl(-) secretion via cAMP signaling and increases cystic fibrosis transmembrane conductance regulator phosphorylation in T84 cells. American Journal of Physiology. Cell Physiology, 305(4), C447-56. https://doi.org/10.1152/ajpcell.00416.2012
Gipson, I. K., Spurr-Michaud, S., Tisdale, A., & Menon, B. B. (2014). Comparison of the transmembrane mucins MUC1 and MUC16 in epithelial barrier function. PloS One, 9(6), e100393. https://doi.org/10.1371/journal.pone.0100393
Othman, R., E Morris, G., Shah, D. A., Hall, S., Hall, G., Wells, K., … Dixon, J. E. (2015). An automated fabrication strategy to create patterned tubular architectures at cell and tissue scales. Biofabrication, 7(2), 025003. https://doi.org/10.1088/1758-5090/7/2/025003
Al-Ghoul, W. M., Kim, M. S., Fazal, N., Azim, A. C., & Ali, A. (2014). Evidence for simvastatin anti-inflammatory actions based on quantitative analyses of NETosis and other inflammation/oxidation markers. Results in Immunology, 4, 14–22. https://doi.org/10.1016/j.rinim.2014.03.001
Katz, J., Sambandam, V., Wu, J. H., Michalek, S. M., & Balkovetz, D. F. (2000). Characterization of Porphyromonas gingivalis-induced degradation of epithelial cell junctional complexes. Infection and Immunity, 68(3), 1441–1449. https://doi.org/10.1128/IAI.68.3.1441-1449.2000
Tsata, V., Velegraki, A., Ioannidis, A., Poulopoulou, C., Bagos, P., Magana, M., & Chatzipanagiotou, S. (2016). Effects of Yeast and Bacterial Commensals and Pathogens of the Female Genital Tract on the Transepithelial Electrical Resistance of HeLa Cells. The Open Microbiology Journal, 10, 90–96. https://doi.org/10.2174/1874285801610010090
Bohara, M., Kambe, Y., Nagayama, T., Tokimura, H., Arita, K., & Miyata, A. (2014). C-type natriuretic peptide modulates permeability of the blood-brain barrier. Journal of Cerebral Blood Flow and Metabolism : Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 34(4), 589–596. https://doi.org/10.1038/jcbfm.2013.234
Pacifico, A., Garnet, A., & Reed, K. (2015). Measuring changes in bioavailability of artemisinin. Major Qualifying Projects (All Years). Retrieved from https://digitalcommons.wpi.edu/mqp-all/229
Gu, M. J., Song, S. K., Lee, I. K., Ko, S., Han, S. E., Bae, S., … Yun, C.-H. (2016). Barrier protection via Toll-like receptor 2 signaling in porcine intestinal epithelial cells damaged by deoxynivalnol. Veterinary Research, 47, 25. https://doi.org/10.1186/s13567-016-0309-1
Keenan, C. R., Mok, J. S., Harris, T., Xia, Y., Salem, S., & Stewart, A. G. (2014). Bronchial epithelial cells are rendered insensitive to glucocorticoid transactivation by transforming growth factor-β1. Respiratory Research, 15(1), 55. https://doi.org/10.1186/1465-9921-15-55
Schneditz, G., Rentner, J., Roier, S., Pletz, J., Herzog, K. A. T., Bücker, R., … Zechner, E. L. (2014). Enterotoxicity of a nonribosomal peptide causes antibiotic-associated colitis. Proceedings of the National Academy of Sciences of the United States of America, 111(36), 13181–13186. https://doi.org/10.1073/pnas.1403274111
Sun, H., Harris, W. T., Kortyka, S., Kotha, K., Ostmann, A. J., Rezayat, A., … Clancy, J. P. (2014). Tgf-beta downregulation of distinct chloride channels in cystic fibrosis-affected epithelia. PloS One, 9(9), e106842. https://doi.org/10.1371/journal.pone.0106842
Ali, M. H., Schlidt, S. A., Chandel, N. S., Hynes, K. L., Schumacker, P. T., & Gewertz, B. L. (1999). Endothelial permeability and IL-6 production during hypoxia: role of ROS in signal transduction. The American Journal of Physiology, 277(5 Pt 1), L1057-65. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10564193
Lea, T. (2015). Epithelial Cell Models; General Introduction. In The Impact of Food Bioactives on Health (pp. 95–102). Cham: Springer International Publishing. https://doi.org/10.1007/978-3-319-16104-4_9
Lever, A. R., Park, H., Mulhern, T. J., Jackson, G. R., Comolli, J. C., Borenstein, J. T., … Prantil-Baun, R. (2015). Comprehensive evaluation of poly(I:C) induced inflammatory response in an airway epithelial model. Physiological Reports, 3(4). https://doi.org/10.14814/phy2.12334
Hellinger, É., Bakk, M. L., Pócza, P., Tihanyi, K., & Vastag, M. (2010). Drug penetration model of vinblastine-treated Caco-2 cultures. European Journal of Pharmaceutical Sciences, 41(1), 96–106. https://doi.org/10.1016/j.ejps.2010.05.015
Hirano, M., & Hirano, K. (2016). Myosin di-phosphorylation and peripheral actin bundle formation as initial events during endothelial barrier disruption. Scientific Reports, 6(1), 20989. https://doi.org/10.1038/srep20989
Schexnayder, C., & Stratford, R. E. (2015). Genistein and Glyceollin Effects on ABCC2 (MRP2) and ABCG2 (BCRP) in Caco-2 Cells. International Journal of Environmental Research and Public Health, 13(1), ijerph13010017. https://doi.org/10.3390/ijerph13010017
Liu, D.-Z., Lecluyse, E. L., & Thakker, D. R. (1999). Dodecylphosphocholine-mediated enhancement of paracellular permeability and cytotoxicity in Caco-2 cell monolayers. Journal of Pharmaceutical Sciences, 88(11), 1161–1168. https://doi.org/10.1021/js990094e
Travanty, E., Zhou, B., Zhang, H., Di, Y. P., Alcorn, J. F., Wentworth, D. E., … Wang, J. (2015). Differential Susceptibilities of Human Lung Primary Cells to H1N1 Influenza Viruses. Journal of Virology, 89(23), 11935–11944. https://doi.org/10.1128/JVI.01792-15
Igarashi, Y., Utsumi, H., Chiba, H., Yamada-Sasamori, Y., Tobioka, H., Kamimura, Y., … Sawada, N. (1999). Glial cell line-derived neurotrophic factor induces barrier function of endothelial cells forming the blood-brain barrier. Biochemical and Biophysical Research Communications, 261(1), 108–112. https://doi.org/10.1006/bbrc.1999.0992
Enjoji, S., Ohama, T., & Sato, K. (2014). Regulation of epithelial cell tight junctions by protease-activated receptor 2. The Journal of Veterinary Medical Science, 76(9), 1225–1229. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24881651
Stanifer, M. L., Rippert, A., Kazakov, A., Willemsen, J., Bucher, D., Bender, S., … Boulant, S. (2016). Reovirus intermediate subviral particles constitute a strategy to infect intestinal epithelial cells by exploiting TGF-β dependent pro-survival signaling. Cellular Microbiology, 18(12), 1831–1845. https://doi.org/10.1111/cmi.12626
Elbakary, B., & Badhan, R. K. S. (2020). A dynamic perfusion based blood-brain barrier model for cytotoxicity testing and drug permeation. Scientific Reports, 10(1), 3788. https://doi.org/10.1038/s41598-020-60689-w
Takakuwa, R., Kokai, Y., Kojima, T., Akatsuka, T., Tobioka, H., Sawada, N., & Mori, M. (2000). Uncoupling of Gate and Fence Functions of MDCK Cells by the Actin-Depolymerizing Reagent Mycalolide B. Experimental Cell Research, 257(2), 238–244. https://doi.org/10.1006/excr.2000.4887
Gallagher, E., Minn, I., Chambers, J. E., & Searson, P. C. (2016). In vitro characterization of pralidoxime transport and acetylcholinesterase reactivation across MDCK cells and stem cell-derived human brain microvascular endothelial cells (BC1-hBMECs). Fluids and Barriers of the CNS, 13(1), 10. https://doi.org/10.1186/s12987-016-0035-0
Curtis, V. F., Ehrentraut, S. F., Campbell, E. L., Glover, L. E., Bayless, A., Kelly, C. J., … Colgan, S. P. (2015). Stabilization of HIF through inhibition of Cullin-2 neddylation is protective in mucosal inflammatory responses. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology, 29(1), 208–215. https://doi.org/10.1096/fj.14-259663
Liu, D. Z., Morris-Natschke, S. L., Kucera, L. S., Ishaq, K. S., Thakker, D. R., Hoogdalem, E. J. Van, … Magnusson, C. (1999). Structure-activity relationships for enhancement of paracellular permeability by 2-alkoxy-3-alkylamidopropylphosphocholines across Caco-2 cell monolayers. Journal of Pharmaceutical Sciences, 88(11), 1169–1174. https://doi.org/10.1021/JS9900957
Ross, B. N.,
7 Reasons to Love the New EVOM3 for TEER Measurement
Great New Features of the EVOM3
The EVOM3 TEER Measurement System enables researchers to carry out experiments more efficiently by improving the workflow, and increasing the stability and accuracy of readings over that of the EVOM2. If you prefer to read the details, see the article "Why Choose an EVOM3 over an EVOM2."
EVOM3: What's New?
Compact and Lightweight - This is a comparison between the EVOM3 and the EVOM2. At less than 1 lb., the EVOM3 is lightweight and portable. It has a sleek design with a touchscreen interface.
Smart Data Display and Foot Switch Control - See how easy it is to setup and use the foot switch to collect data.
Data Storage on USB Flash Drive - Save data as a Microsoft Excel files on the USB flash drive with the touch of a button. The data file can be accessed on a computer by plugging in the flash drive to a USB port.
Improved Electrode Design - Compare the STX2 electrode and the NEW STX2-PLUS electrode. The new electrode stands vertically on the well plate, ensuring stable and consistent readings.