This website uses cookies to ensure you get the best experience on our website.
Read more
Epithelial Volt/Ohm (TEER) Meter - DISCONTINUED
$5,125.00
Prices valid in USA, Canada, and PR only.
Order code
EVOM2

Prices valid in USA, Canada, and PR only.
This product has been discontinued. UPGRADE TO EVOM™ MANUAL for increased accuracy, auto data logging, touchscreen interface, and many new features.
EVOM™ Manual replaces all WPI-manufactured manual TEER meters, including EVOM3, EVOM2 and MilliCell® ERS-2, all of which have been discontinued.
Prices valid in USA, Canada, and PR only.
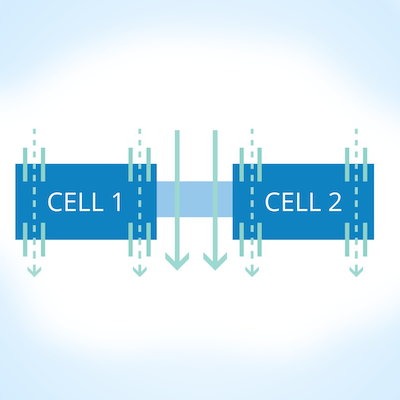
Non-destructively test for epithelial monolayer confluence in 2D cell cultures
- Measures trans-epithelial electrical resistance or trans-epithelilal voltage
- Compatible with 12 and 24 well culture plate systems out of the box
- Includes industry standard STX2 hand held “chopstick” electrodes
- Analog output for recording resistance or voltage measurements
- Auto ranging from 0-10 KΩ
- Battery powered
- Manual TEER measurement of epithelial cells in 6, 12, 24 and 96* well plates
- BNC output for data acquisition system
- Compatible with EndOhm chambers
- *96-well plate measurement requires an STX100 series electrode.
Click here to view the current Data Sheet.
Options
| Order code | Voltmeter | Electrode | Charger (800496) |
Battery (91736) |
1 k-Ω Test Resistor (91750) |
| EVOM2 | 0-10 kΩ Range Epithelial Volt/Ohm Meter |
(Basic Electrode) |
Yes | Yes (Installed) | Yes |
| 91799 | 0-10 kΩ Range Epithelial Volt/Ohm Meter |
STX3 |
Yes | Yes (Installed) | Yes |
| 300523 | 0-100 kΩ Range Epithelial Volt/Ohm Meter |
(Basic Electrode) |
Yes | Yes (Installed) | Yes |
Benefits
- You can verify performance and calibrate the meter for TEER function using provided test resistor
- Battery powered meter is portable
- A variety of accessory electrodes are available for measuring TEER in 6- and 96-well fixed (HTS) and removable well culture systems (See STX100 series and Endohm electrodes)
Applications
- TEER and trans-epithelial voltage measurements in 2D cell cultures
Making TEER Measurements to Determine Cellular Confluence
The EVOM was the first instrument designed specifically to perform routine Trans Epithelial Electrical Resistance (TEER) measurement in tissue culture research. EVOM2™ is the next generation, redesigned for ease of use. The EVOM2™ not only qualitatively measures cell monolayer health, but also quantitatively measures cellular confluence. The unique electronic circuit of the EVOM2™ and the included STX2 electrode detect the confluence of the cellular monolayer. When combined with WPI’s Endohm chamber, the EVOM2™ can also be used to perform more accurate quantitative measurements or lower resistance measurements like transendothelial electrical resistance measurements.
Isolated battery power for 10 hours of use
The isolated power source of the EVOM2™ was specifically designed to avoid adverse effects on tissue and the formation of electrode metal deposits, even when it is plugged into a standard wall outlet. Now, the EVOM2™ is always on when you need it. In addition, its rechargeable battery allows up to 10 hours of mobile use.
Accurate reading every time
The four-and-a-half digit readout provides a range of 1-9,999 Ω. The included test electrode lets you calibrate the resistance measurements for an accurate reading every time. An analog BNC output is standard with the EVOM2™, providing an output port for recording data or remote display of the EVOM2™ output.
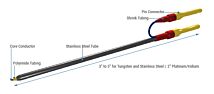
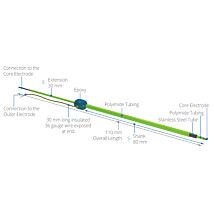
Electrode pair to measure voltage, pass current
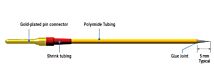
EVOM2™ comes complete with the popular STX2 “chopstick” electrodes, 4 mm wide and 1 mm thick. Each stick of the electrode pair contains a silver/silver-chloride pellet for measuring voltage and a silver electrode for passing current. The small size of each electrode is designed to facilitate placement of the electrodes into a variety of standard cell culture wells.


STX2 STX3
| SKU | EVOM2 |
|---|---|
| Options | Resistance Range 0-10 KΩ with STX2 |
Upsell Products
- $295.00
- $52.00
-
Cell Culture Cup Chambers for TEER Measurement
As low as $2,250.00 - $2,250.00
-
CHOPSTICKS ELECTRODE SET FOR EVOM
Request Quote
Video
VIDEO: Models and Methods to Evaluate Transport of Drug Delivery Systems Across Cellular Barriers
See how to use and EndOhm chamber with an EVOM2 meter.
In this video Mike shows you how to equilibrate your STX electrodes.
In this video, you can learn how to test your EVOM2.
Cellular Confluency of Epithelial Cells
The original EVOM was featured in this application video.
FAQ
- Will the EVOM3 work with Endohm’s?
Yes, but the 99672 adaptor is required or the new EVOM3 cable 99916.
- Why would I want to use the blank function?
The blank feature is used when you want to subtract out any measurement that is not from the membrane, such as the electrode and fluid resistances.
- Does the EVOM3 system automatically calculate TEER?
No, TEER measurement requires an area calculation. To compute TEER, divide the measured resistance by the appropriate surface area (below). For example, a 12 mm insert measures 565 Ω, the TEER is 565 Ω/1.13 or 500 Ω. 6 well plate (24 mm inserts) 4.53 cm2, 12 well plate (12 mm inserts) 1.13 cm2, 24 well plate 6.5 mm inserts) 0.3316 cm2, 96 well plate (4.3 mm inserts) 0.143 cm2.
- EVOM3 data is stored automatically when the last well is reached. How do I store the data when I only want to measure 8 of 96 wells?
Clear any data in memory by opening settings, store menu then press new plate, that will clear any prior readings. Return to the main screen, open the preview screen, select each well to measure (the selection turns green), place the electrode, then measure. When you’re done measuring the selected wells, open the settings, press the store screen menu, then press store new to save the plate data to the USB drive.
| Membrane Voltage Range | +/-200 mv |
| Resolution | 0.1 mV |
| Resistance Range | 0 to 9999 Ω * |
| Resistance Resolution | 1 Ω |
| AC Square Wave Current | +/- 10uA nominal at 12.5 Hz |
| Power | Internal rechargeable 6V NiMH 2700 mAH batter with external 12VDC Supply for recharging |
| Nomimal Battery Run Time | 10 hours |
| BNC Output |
1-10 V (1 mV/Ω)
|
| Dimensions | 19x11x6 cm (7.25x4.25x2.30") |
| Weight | 1.4 kg (3 lb.) |
| Electrode Connection | RJ-11 connector (telephone style) |
| Test Resistor | External, 1000 Ω |
| Environmental Range | 10-38°C (50-100°F) 0-90% non-condensing relative humidity |
| Power Supply | Universal 100-240 VAC, 120 VDC (5.5 x 2.5mm barrel positive tip), 850 mA |
* 300523 is the part number for the EVOM2 with 10X the resistance range. Note that display on this unit reads in KΩ, and the display on the standard EVOM2 read Ω.
Gomez-Martinez, I., Jarrett Bliton, R., Breau, K. A., Czerwinski, M. J., Williamson, I. A., Wen, J., Rawls, J. F., & Magness, S. T.(2022). ORIGINAL RESEARCH A Planar Culture Model of Human Absorptive Enterocytes Reveals Metformin Increases Fatty Acid Oxidation and Export 96-well Format Fluorescent Fatty Acids BODIPY-C12 BODIPY-C16. Cellular and Molecular Gastroenterology and Hepatology, 14, 409–434. https://doi.org/10.1016/j.jcmgh.2022.04.009
Elbakary, B., & Badhan, R. K. S. (2020). A dynamic perfusion based blood-brain barrier model for cytotoxicity testing and drug permeation. Scientific Reports, 10(1), 3788. https://doi.org/10.1038/s41598-020-60689-w
Neal, E. H., Marinelli, N. A., Shi, Y., McClatchey, P. M., Balotin, K. M., Gullett, D. R., … Lippmann, E. S. (2019). A Simplified, Fully Defined Differentiation Scheme for Producing Blood-Brain Barrier Endothelial Cells from Human iPSCs. Stem Cell Reports, 12(6), 1380–1388. https://doi.org/10.1016/J.STEMCR.2019.05.008
Dickman, K. G., Hempson, S. J., Anderson, J., Lippe, S., Zhao, L., Burakoff, R., & Shaw, R. D. (n.d.). Rotavirus alters paracellular permeability and energy metabolism in Caco-2 cells.
Patch-clamp technique. (n.d.).
Sawai, T., Usui, N., Dwaihy, J., Drongowski, R. A., Abe, A., Coran, A. G., & Harmon, C. M. (n.d.). The effect of phospholipase A2 on bacterial translocation in a cell culture model. Pediatric Surgery International, 16(4), 262–266. https://doi.org/10.1007/S003830050741
Maherally, Z., Fillmore, H. L., Tan, S. L., Tan, S. F., Jassam, S. A., Quack, F. I., … Pilkington, G. J. (2018). Real‐time acquisition of transendothelial electrical resistance in an all‐human, in vitro , 3‐dimensional, blood‐brain barrier model exemplifies tight‐junction integrity. The FASEB Journal, 32(1), 168–182. https://doi.org/10.1096/fj.201700162R
Pham, V. T., Seifert, N., Richard, N., Raederstorff, D., Steinert, R., Prudence, K., & Mohajeri, M. H. (2018). The effects of fermentation products of prebiotic fibres on gut barrier and immune functions in vitro. PeerJ, 6, e5288. https://doi.org/10.7717/peerj.5288
Hollmann, E. K., Bailey, A. K., Potharazu, A. V., Neely, M. D., Bowman, A. B., & Lippmann, E. S. (2017). Accelerated differentiation of human induced pluripotent stem cells to blood–brain barrier endothelial cells. Fluids and Barriers of the CNS 2017 14:1, 14(1), 1–13. https://doi.org/10.1186/S12987-017-0059-0
Sheller, R. A., Cuevas, M. E., & Todd, M. C. (2017). Comparison of transepithelial resistance measurement techniques: Chopsticks vs. Endohm. Biological Procedures Online, 19, 4. https://doi.org/10.1186/s12575-017-0053-6
Sei, Y. J., Ahn, S. I., Virtue, T., Kim, T., & Kim, Y. (2017). Detection of frequency-dependent endothelial response to oscillatory shear stress using a microfluidic transcellular monitor. Scientific Reports, 7(1), 10019. https://doi.org/10.1038/s41598-017-10636-z
Shang, V. C. M., Kendall, D. A., & Roberts, R. E. (2016). Δ9-Tetrahydrocannabinol reverses TNFα-induced increase in airway epithelial cell permeability through CB2 receptors. Biochemical Pharmacology, 120, 63–71. https://doi.org/10.1016/j.bcp.2016.09.008
Stanifer, M. L., Rippert, A., Kazakov, A., Willemsen, J., Bucher, D., Bender, S., … Boulant, S. (2016). Reovirus intermediate subviral particles constitute a strategy to infect intestinal epithelial cells by exploiting TGF-β dependent pro-survival signaling. Cellular Microbiology, 18(12), 1831–1845. https://doi.org/10.1111/cmi.12626
Jacobs, M. E., Kathpalia, P. P., Chen, Y., Thomas, S. V., Noonan, E. J., & Pao, A. C. (2016). SGK1 regulation by miR-466g in cortical collecting duct cells. American Journal of Physiology-Renal Physiology, 310(11), F1251–F1257. https://doi.org/10.1152/ajprenal.00024.2016
Meenach, S. A., Tsoras, A. N., McGarry, R. C., Mansour, H. M., Hilt, J. Z., & Anderson, K. W. (2016). Development of three-dimensional lung multicellular spheroids in air- and liquid-interface culture for the evaluation of anticancer therapeutics. International Journal of Oncology, 48(4), 1701–1709. https://doi.org/10.3892/ijo.2016.3376
Mansley, M. K., Watt, G. B., Francis, S. L., Walker, D. J., Land, S. C., Bailey, M. A., & Wilson, S. M. (2016). Dexamethasone and insulin activate serum and glucocorticoid-inducible kinase 1 (SGK1) via different molecular mechanisms in cortical collecting duct cells. Physiological Reports, 4(10). https://doi.org/10.14814/phy2.12792
Rahman, N. A., Rasil, A. N. H. M., Meyding-Lamade, U., Craemer, E. M., Diah, S., Tuah, A. A., & Muharram, S. H. (2016). Immortalized endothelial cell lines for in vitro blood–brain barrier models: A systematic review. Brain Research, 1642, 532–545. https://doi.org/10.1016/j.brainres.2016.04.024
Gu, M. J., Song, S. K., Lee, I. K., Ko, S., Han, S. E., Bae, S., … Yun, C.-H. (2016). Barrier protection via Toll-like receptor 2 signaling in porcine intestinal epithelial cells damaged by deoxynivalnol. Veterinary Research, 47, 25. https://doi.org/10.1186/s13567-016-0309-1
Gallagher, E., Minn, I., Chambers, J. E., & Searson, P. C. (2016). In vitro characterization of pralidoxime transport and acetylcholinesterase reactivation across MDCK cells and stem cell-derived human brain microvascular endothelial cells (BC1-hBMECs). Fluids and Barriers of the CNS, 13(1), 10. https://doi.org/10.1186/s12987-016-0035-0
Iacovelli, J., Rowe, G. C., Khadka, A., Diaz-Aguilar, D., Spencer, C., Arany, Z., & Saint-Geniez, M. (2016). PGC-1α Induces Human RPE Oxidative Metabolism and Antioxidant Capacity. Investigative Ophthalmology & Visual Science, 57(3), 1038–1051. https://doi.org/10.1167/iovs.15-17758
Richter, J. F., Schmauder, R., Krug, S. M., Gebert, A., & Schumann, M. (2016). A novel method for imaging sites of paracellular passage of macromolecules in epithelial sheets. Journal of Controlled Release, 229, 70–79. https://doi.org/10.1016/j.jconrel.2016.03.018
Griffin, J. M., Kho, D., Graham, E. S., Nicholson, L. F. B., & O’Carroll, S. J. (2016). Statins Inhibit Fibrillary β-Amyloid Induced Inflammation in a Model of the Human Blood Brain Barrier. PloS One, 11(6), e0157483. https://doi.org/10.1371/journal.pone.0157483
Fiandra, L., Mazzucchelli, S., Truffi, M., Bellini, M., Sorrentino, L., & Corsi, F. (2016). <em>In Vitro</em> Permeation of FITC-loaded Ferritins Across a Rat Blood-brain Barrier: a Model to Study the Delivery of Nanoformulated Molecules. Journal of Visualized Experiments, (114), e54279–e54279. https://doi.org/10.3791/54279
Yan, Y., Shapiro, A. P., Mopidevi, B. R., Chaudhry, M. A., Maxwell, K., Haller, S. T., … Liu, J. (2016). Protein Carbonylation of an Amino Acid Residue of the Na/K-ATPase α1 Subunit Determines Na/K-ATPase Signaling and Sodium Transport in Renal Proximal Tubular Cells. Journal of the American Heart Association, 5(9). https://doi.org/10.1161/JAHA.116.003675
Torr, E., Heath, M., Mee, M., Shaw, D., Sharp, T. V, & Sayers, I. (2016). Expression of polycomb protein BMI-1 maintains the plasticity of basal bronchial epithelial cells. Physiological Reports, 4(16). https://doi.org/10.14814/phy2.12847
Wardill, H. R., Gibson, R. J., Van Sebille, Y. Z., Secombe, K. R., Logan, R. M., & Bowen, J. M. (2016). A novel in vitro platform for the study of SN38-induced mucosal damage and the development of Toll-like receptor 4-targeted therapeutic options. Experimental Biology and Medicine, 241(13), 1386–1394. https://doi.org/10.1177/1535370216640932
Slater, M., Torr, E., Harrison, T., Forrester, D., Knox, A., Shaw, D., & Sayers, I. (2016). The differential effects of azithromycin on the airway epithelium in vitro and in vivo. Physiological Reports, 4(18). https://doi.org/10.14814/phy2.12960
Miao, W., Wu, X., Wang, K., Wang, W., Wang, Y., Li, Z., … Peng, L. (2016). Sodium Butyrate Promotes Reassembly of Tight Junctions in Caco-2 Monolayers Involving Inhibition of MLCK/MLC2 Pathway and Phosphorylation of PKCβ2. International Journal of Molecular Sciences, 17(10). https://doi.org/10.3390/ijms17101696
Melvin, J. A., Lashua, L. P., Kiedrowski, M. R., Yang, G., Deslouches, B., Montelaro, R. C., & Bomberger, J. M. (2016). Simultaneous Antibiofilm and Antiviral Activities of an Engineered Antimicrobial Peptide during Virus-Bacterium Coinfection. MSphere, 1(3). https://doi.org/10.1128/mSphere.00083-16
Tosoni, K., Cassidy, D., Kerr, B., Land, S. C., & Mehta, A. (2016). Using Drugs to Probe the Variability of Trans-Epithelial Airway Resistance. PloS One, 11(2), e0149550. https://doi.org/10.1371/journal.pone.0149550
Hirano, M., & Hirano, K. (2016). Myosin di-phosphorylation and peripheral actin bundle formation as initial events during endothelial barrier disruption. Scientific Reports, 6(1), 20989. https://doi.org/10.1038/srep20989
Turdalieva, A., Solandt, J., Shambetova, N., Xu, H., Blom, H., Brismar, H., … Fu, Y. (2016). Bioelectric and Morphological Response of Liquid-Covered Human Airway Epithelial Calu-3 Cell Monolayer to Periodic Deposition of Colloidal 3-Mercaptopropionic-Acid Coated CdSe-CdS/ZnS Core-Multishell Quantum Dots. PloS One, 11(2), e0149915. https://doi.org/10.1371/journal.pone.0149915
Williams, K. M., Gokulan, K., Cerniglia, C. E., & Khare, S. (2016). Size and dose dependent effects of silver nanoparticle exposure on intestinal permeability in an in vitro model of the human gut epithelium. Journal of Nanobiotechnology, 14(1), 62. https://doi.org/10.1186/s12951-016-0214-9
Bartakova, A., Alvarez-Delfin, K., Weisman, A. D., Salero, E., Raffa, G. A., Merkhofer, R. M., … Goldberg, J. L. (2016). Novel Identity and Functional Markers for Human Corneal Endothelial Cells. Investigative Ophthalmology & Visual Science, 57(6), 2749–2762. https://doi.org/10.1167/iovs.15-18826
Tsata, V., Velegraki, A., Ioannidis, A., Poulopoulou, C., Bagos, P., Magana, M., & Chatzipanagiotou, S. (2016). Effects of Yeast and Bacterial Commensals and Pathogens of the Female Genital Tract on the Transepithelial Electrical Resistance of HeLa Cells. The Open Microbiology Journal, 10, 90–96. https://doi.org/10.2174/1874285801610010090
Marvin, S. A., Huerta, C. T., Sharp, B., Freiden, P., Cline, T. D., & Schultz-Cherry, S. (2016). Type I Interferon Response Limits Astrovirus Replication and Protects against Increased Barrier Permeability In Vitro and In Vivo. Journal of Virology, 90(4), 1988–1996. https://doi.org/10.1128/JVI.02367-15
Netsomboon, K., Laffleur, F., & Bernkop-Schnürch, A. (2016). P-glycoprotein inhibitors: synthesis and in vitro evaluation of a preactivated thiomer. Drug Development and Industrial Pharmacy, 42(4), 668–675. https://doi.org/10.3109/03639045.2015.1075025
Lewis, S. B., Prior, A., Ellis, S. J., Cook, V., Chan, S. S. M., Gelson, W., & Schüller, S. (2016). Flagellin Induces β-Defensin 2 in Human Colonic Ex vivo Infection with Enterohemorrhagic Escherichia coli. Frontiers in Cellular and Infection Microbiology, 6, 68. https://doi.org/10.3389/fcimb.2016.00068
Paradis, A., Leblanc, D., & Dumais, N. (2016). Optimization of an in vitro human blood-brain barrier model: Application to blood monocyte transmigration assays. MethodsX, 3, 25–34. https://doi.org/10.1016/j.mex.2015.11.009
Bernocchi, B., Carpentier, R., Lantier, I., Ducournau, C., Dimier-Poisson, I., & Betbeder, D. (2016). Mechanisms allowing protein delivery in nasal mucosa using NPL nanoparticles. Journal of Controlled Release : Official Journal of the Controlled Release Society, 232, 42–50. https://doi.org/10.1016/j.jconrel.2016.04.014
Ahmed, C. M., Biswal, M. R., Li, H., Han, P., Ildefonso, C. J., & Lewin, A. S. (2016). Repurposing an orally available drug for the treatment of geographic atrophy. Molecular Vision, 22, 294–310. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/27110092
Davis, B. P., Stucke, E. M., Khorki, M. E., Litosh, V. A., Rymer, J. K., Rochman, M., … Orlando, R. (2016). Eosinophilic esophagitis–linked calpain 14 is an IL-13–induced protease that mediates esophageal epithelial barrier impairment. JCI Insight, 1(4), 895–900. https://doi.org/10.1172/jci.insight.86355
Pastor-Clerigues, A., Serrano, A., Milara, J., Marti-Bonmati, E., Lopez-Perez, F. J., Garcia-Montanes, S., … Cortijo, J. (2016). Evaluation of the Ocular Tolerance of Three Tacrolimus Topical Pharmaceutical Preparations by Bovine Corneal Opacity and Permeability Test. Current Eye Research, 41(7), 890–896. https://doi.org/10.3109/02713683.2015.1082187
Srimanee, A., Regberg, J., Hällbrink, M., Vajragupta, O., & Langel, Ü. (2016). Role of scavenger receptors in peptide-based delivery of plasmid DNA across a blood–brain barrier model. International Journal of Pharmaceutics, 500(1–2), 128–135. https://doi.org/10.1016/j.ijpharm.2016.01.014
Byeon, H. J., Thao, L. Q., Lee, S., Min, S. Y., Lee, E. S., Shin, B. S., … Youn, Y. S. (2016). Doxorubicin-loaded nanoparticles consisted of cationic- and mannose-modified-albumins for dual-targeting in brain tumors. Journal of Controlled Release, 225, 301–313. https://doi.org/10.1016/j.jconrel.2016.01.046
Di, S., Gujie, M., & Thomas, W. (2016). Magnetic ferri-liposomes for triggered drug release across the blood- brain barrier. Frontiers in Bioengineering and Biotechnology, 4. https://doi.org/10.3389/conf.FBIOE.2016.01.00061
Ross, B. N., Rojas-Lopez, M., Cieza, R. J., McWilliams, B. D., & Torres, A. G. (2015). The Role of Long Polar Fimbriae in Escherichia coli O104:H4 Adhesion and Colonization. PLOS ONE, 10(10), e0141845. https://doi.org/10.1371/journal.pone.0141845
Travanty, E., Zhou, B., Zhang, H., Di, Y. P., Alcorn, J. F., Wentworth, D. E., … Wang, J. (2015). Differential Susceptibilities of Human Lung Primary Cells to H1N1 Influenza Viruses. Journal of Virology, 89(23), 11935–11944. https://doi.org/10.1128/JVI.01792-15
Loma, P., Guzman-Aranguez, A., Pérez de Lara, M. J., & Pintor, J. (2015). Diadenosine tetraphosphate induces tight junction disassembly thus increasing corneal epithelial permeability. British Journal of Pharmacology, 172(4), 1045–1058. https://doi.org/10.1111/bph.12972
Prewitt, A. R., Ghose, S., Frump, A. L., Datta, A., Austin, E. D., Kenworthy, A. K., & de Caestecker, M. P. (2015). Heterozygous null bone morphogenetic protein receptor type 2 mutations promote SRC kinase-dependent caveolar trafficking defects and endothelial dysfunction in pulmonary arterial hypertension. The Journal of Biological Chemistry, 290(2), 960–971. https://doi.org/10.1074/jbc.M114.591057
Lewis, S. B., Cook, V., Tighe, R., & Schüller, S. (2015). Enterohemorrhagic Escherichia coli colonization of human colonic epithelium in vitro and ex vivo. Infection and Immunity, 83(3), 942–949. https://doi.org/10.1128/IAI.02928-14
Blenkinsop, T. A., Saini, J. S., Maminishkis, A., Bharti, K., Wan, Q., Banzon, T., … Stern, J. H. (2015). Human Adult Retinal Pigment Epithelial Stem Cell-Derived RPE Monolayers Exhibit Key Physiological Characteristics of Native Tissue. Investigative Ophthalmology & Visual Science, 56(12), 7085–7099. https://doi.org/10.1167/iovs.14-16246
Curtis, V. F., Ehrentraut, S. F., Campbell, E. L., Glover, L. E., Bayless, A., Kelly, C. J., … Colgan, S. P. (2015). Stabilization of HIF through inhibition of Cullin-2 neddylation is protective in mucosal inflammatory responses. The FASEB Journal, 29(1), 208–215. https://doi.org/10.1096/fj.14-259663
Patella, F., Schug, Z. T., Persi, E., Neilson, L. J., Erami, Z., Avanzato, D., … Zanivan, S. (2015). Proteomics-based metabolic modeling reveals that fatty acid oxidation (FAO) controls endothelial cell (EC) permeability. Molecular & Cellular Proteomics : MCP, 14(3), 621–634. https://doi.org/10.1074/mcp.M114.045575
Li, Q., Chen, B., Zeng, C., Fan, A., Yuan, Y., Guo, X., … Huang, Q. (2015). Differential activation of receptors and signal pathways upon stimulation by different doses of sphingosine-1-phosphate in endothelial cells. Experimental Physiology, 100(1), 95–107. https://doi.org/10.1113/expphysiol.2014.082149
Baddal, B., Muzzi, A., Censini, S., Calogero, R. A., Torricelli, G., Guidotti, S., … Pezzicoli, A. (2015). Dual RNA-seq of Nontypeable Haemophilus influenzae and Host Cell Transcriptomes Reveals Novel Insights into Host-Pathogen Cross Talk. MBio, 6(6), e01765-15. https://doi.org/10.1128/mBio.01765-15
Yu, C., Jia, G., Deng, Q., Zhao, H., Chen, X., Liu, G., & Wang, K. (2015). The Effects of Glucagon-like Peptide-2 on the Tight Junction and Barrier Function in IPEC-J2 Cells through Phosphatidylinositol 3-kinase–Protein Kinase B–Mammalian Target of Rapamycin Signaling Pathway. Asian-Australasian Journal of Animal Sciences, 29(5), 731–738. https://doi.org/10.5713/ajas.15.0415
Datta, P., & Weis, M. T. (2015). Calcium glycerophosphate preserves transepithelial integrity in the Caco-2 model of intestinal transport. World Journal of Gastroenterology, 21(30), 9055–9066. https://doi.org/10.3748/wjg.v21.i30.9055
Ferguson, M. C., Saul, S., Fragkoudis, R., Weisheit, S., Cox, J., Patabendige, A., … Fazakerley, J. K. (2015). Ability of the Encephalitic Arbovirus Semliki Forest Virus To Cross the Blood-Brain Barrier Is Determined by the Charge of the E2 Glycoprotein. Journal of Virology, 89(15), 7536–7549. https://doi.org/10.1128/JVI.03645-14
Chen, S., Einspanier, R., & Schoen, J. (2015). Transepithelial electrical resistance (TEER): a functional parameter to monitor the quality of oviduct epithelial cells cultured on filter supports. Histochemistry and Cell Biology, 144(5), 509–515. https://doi.org/10.1007/s00418-015-1351-1
Zhang, J., Ni, C., Yang, Z., Piontek, A., Chen, H., Wang, S., … Piontek, J. (2015). Specific binding of Clostridium perfringens enterotoxin fragment to Claudin-b and modulation of zebrafish epidermal barrier. Experimental Dermatology, 24(8), 605–610. https://doi.org/10.1111/exd.12728
Leir, S.-H., Browne, J. A., Eggener, S. E., & Harris, A. (2015). Characterization of primary cultures of adult human epididymis epithelial cells. Fertility and Sterility, 103(3), 647–54.e1. https://doi.org/10.1016/j.fertnstert.2014.11.022
Ma, W., Feng, S., Yao, X., Yuan, Z., Liu, L., & Xie, Y. (2015). Nobiletin enhances the efficacy of chemotherapeutic agents in ABCB1 overexpression cancer cells. Scientific Reports, 5, 18789. https://doi.org/10.1038/srep18789
Pothoven, K. L., Norton, J. E., Hulse, K. E., Suh, L. A., Carter, R. G., Rocci, E., … Schleimer, R. P. (2015). Oncostatin M promotes mucosal epithelial barrier dysfunction, and its expression is increased in patients with eosinophilic mucosal disease. The Journal of Allergy and Clinical Immunology, 136(3), 737–746.e4. https://doi.org/10.1016/j.jaci.2015.01.043
Othman, R., E Morris, G., Shah, D. A., Hall, S., Hall, G., Wells, K., … Dixon, J. E. (2015). An automated fabrication strategy to create patterned tubular architectures at cell and tissue scales. Biofabrication, 7(2), 025003. https://doi.org/10.1088/1758-5090/7/2/025003
Lea, T. (2015). Epithelial Cell Models; General Introduction. In The Impact of Food Bioactives on Health (pp. 95–102). Cham: Springer International Publishing. https://doi.org/10.1007/978-3-319-16104-4_9
Park, S. W., Kim, J. H., Park, S. M., Moon, M., Lee, K. H., Park, K. H., … Kim, J. H. (2015). RAGE mediated intracellular Aβ uptake contributes to the breakdown of tight junction in retinal pigment epithelium. Oncotarget, 6(34), 35263–35273. https://doi.org/10.18632/oncotarget.5894
Loma, P., Guzman-Aranguez, A., Pérez de Lara, M. J., & Pintor, J. (2015). Diadenosine tetraphosphate induces tight junction disassembly thus increasing corneal epithelial permeability. British Journal of Pharmacology, 172(4), 1045–1058. https://doi.org/10.1111/bph.12972
Erami, Z., Timpson, P., Yao, W., Zaidel-Bar, R., & Anderson, K. I. (2015). There are four dynamically and functionally distinct populations of E-cadherin in cell junctions. Biology Open, 4(11), 1481–1489. https://doi.org/10.1242/bio.014159
Lin, B., Liu, Y., Li, T., Zeng, K., Cai, S., Zeng, Z., … Gao, Y. (2015). Ulinastatin mediates protection against vascular hyperpermeability following hemorrhagic shock. International Journal of Clinical and Experimental Pathology, 8(7), 7685–7693. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/26339335
Roos, S., Wyder, M., Candi, A., Regenscheit, N., Nathues, C., van Immerseel, F., & Posthaus, H. (2015). Binding studies on isolated porcine small intestinal mucosa and in vitro toxicity studies reveal lack of effect of C. perfringens beta-toxin on the porcine intestinal epithelium. Toxins, 7(4), 1235–1252. https://doi.org/10.3390/toxins7041235
Venter, J., Francis, H., Meng, F., DeMorrow, S., Kennedy, L., Standeford, H., … Alpini, G. (2015). Development and functional characterization of extrahepatic cholangiocyte lines from normal rats. Digestive and Liver Disease : Official Journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver, 47(11), 964–972. https://doi.org/10.1016/j.dld.2015.07.012
Odijk, M., van der Meer, A. D., Levner, D., Kim, H. J., van der Helm, M. W., Segerink, L. I., … van den Berg, A. (2015). Measuring direct current trans-epithelial electrical resistance in organ-on-a-chip microsystems. Lab on a Chip, 15(3), 745–752. https://doi.org/10.1039/c4lc01219d
Mooren, O. L., Kim, J., Li, J., & Cooper, J. A. (2015). Role of N-WASP in Endothelial Monolayer Formation and Integrity. Journal of Biological Chemistry, 290(30), 18796–18805. https://doi.org/10.1074/jbc.M115.668285
Noh, S. Y., Kang, S.-S., Yun, C.-H., & Han, S. H. (2015). Lipoteichoic acid from Lactobacillus plantarum inhibits Pam2CSK4-induced IL-8 production in human intestinal epithelial cells. Molecular Immunology, 64(1), 183–189. https://doi.org/10.1016/j.molimm.2014.11.014
Deosarkar, S. P., Prabhakarpandian, B., Wang, B., Sheffield, J. B., Krynska, B., & Kiani, M. F. (2015). A Novel Dynamic Neonatal Blood-Brain Barrier on a Chip. PloS One, 10(11), e0142725. https://doi.org/10.1371/journal.pone.0142725
Mladenova, K., Petrova, S., Moskova-Doumanova, V., Topouzova-Hristova, T., Stoitsova, S., Tabashka, I., … Doumanov, J. (2015). Transepithelial resistance in human bestrophin-1 stably transfected Madin-Darby canine kidney cells. Biotechnology, Biotechnological Equipment, 29(1), 101–104. https://doi.org/10.1080/13102818.2014.988078
Zhang, J., Field, C. J., Vine, D., & Chen, L. (2015). Intestinal Uptake and Transport of Vitamin B12-loaded Soy Protein Nanoparticles. Pharmaceutical Research, 32(4), 1288–1303. https://doi.org/10.1007/s11095-014-1533-x
Xing, F., Sharma, S., Liu, Y., Mo, Y.-Y., Wu, K., Zhang, Y.-Y., … Watabe, K. (2015). miR-509 suppresses brain metastasis of breast cancer cells by modulating RhoC and TNF-α. Oncogene, 34(37), 4890–4900. https://doi.org/10.1038/onc.2014.412
Liu, Z., Zhang, F., Koh, G. Y., Dong, X., Hollingsworth, J., Zhang, J., … Stout, R. W. (2015). Cytotoxic and antiangiogenic paclitaxel solubilized and permeation-enhanced by natural product nanoparticles. Anti-Cancer Drugs, 26(2), 167–179. https://doi.org/10.1097/CAD.0000000000000173
Lever, A. R., Park, H., Mulhern, T. J., Jackson, G. R., Comolli, J. C., Borenstein, J. T., … Prantil-Baun, R. (2015). Comprehensive evaluation of poly(I:C) induced inflammatory response in an airway epithelial model. Physiological Reports, 3(4). https://doi.org/10.14814/phy2.12334
Srinivasan, B., Kolli, A. R., Esch, M. B., Abaci, H. E., Shuler, M. L., & Hickman, J. J. (2015). TEER measurement techniques for in vitro barrier model systems. Journal of Laboratory Automation, 20(2), 107–126. https://doi.org/10.1177/2211068214561025
Zaccone, E. J., Goldsmith, W. T., Shimko, M. J., Wells, J. R., Schwegler-Berry, D., Willard, P. A., … Fedan, J. S. (2015). Diacetyl and 2,3-pentanedione exposure of human cultured airway epithelial cells: Ion transport effects and metabolism of butter flavoring agents. Toxicology and Applied Pharmacology, 289(3), 542–549. https://doi.org/10.1016/j.taap.2015.10.004
Cao, X., Lin, H., Muskhelishvili, L., Latendresse, J., Richter, P., Heflich, R. H., … Browning, M. (2015). Tight junction disruption by cadmium in an in vitro human airway tissue model, 16(1), 30. https://doi.org/10.1186/s12931-015-0191-9
Curtis, V. F., Ehrentraut, S. F., Campbell, E. L., Glover, L. E., Bayless, A., Kelly, C. J., … Colgan, S. P. (2015). Stabilization of HIF through inhibition of Cullin-2 neddylation is protective in mucosal inflammatory responses. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology, 29(1), 208–215. https://doi.org/10.1096/fj.14-259663
Le, M. T., van Veldhuizen, M., Porcelli, I., Bongaerts, R. J., Gaskin, D. J. H., Pearson, B. M., & van Vliet, A. H. M. (2015). Conservation of σ28-Dependent Non-Coding RNA Paralogs and Predicted σ54-Dependent Targets in Thermophilic Campylobacter Species. PloS One, 10(10), e0141627. https://doi.org/10.1371/journal.pone.0141627
Ross, B. N., Rojas-Lopez, M., Cieza, R. J., McWilliams, B. D., & Torres, A. G. (2015). The Role of Long Polar Fimbriae in Escherichia coli O104:H4 Adhesion and Colonization. PloS One, 10(10), e0141845. https://doi.org/10.1371/journal.pone.0141845
Qosa, H., Batarseh, Y. S., Mohyeldin, M. M., El Sayed, K. A., Keller, J. N., & Kaddoumi, A. (2015). Oleocanthal Enhances Amyloid-β Clearance from the Brains of TgSwDI Mice and in Vitro across a Human Blood-Brain Barrier Model. ACS Chemical Neuroscience, 6(11), 1849–1859. https://doi.org/10.1021/acschemneuro.5b00190
Marvin, S. A., Huerta, C. T., Sharp, B., Freiden, P., Cline, T. D., & Schultz-Cherry, S. (2015). Type I Interferon Response Limits Astrovirus Replication and Protects against Increased Barrier Permeability In Vitro and In Vivo. Journal of Virology, 90(4), 1988–1996. https://doi.org/10.1128/JVI.02367-15
Senyavina, N. V., & Tonevitskaya, S. A. (2015). Effect of Hypoxanthine on Functional Activity of Nucleoside Transporters ENT1 and ENT2 in Caco-2 Polar Epithelial Intestinal Cells. Bulletin of Experimental Biology and Medicine, 160(1), 160–164. https://doi.org/10.1007/s10517-015-3118-z
Xu, Q., Liu, J., Wang, Z., Guo, X., Zhou, G., Liu, Y., … Su, L. (2015). Heat stress-induced disruption of endothelial barrier function is via PAR1 signaling and suppressed by Xuebijing injection. PloS One, 10(2), e0118057. https://doi.org/10.1371/journal.pone.0118057
Brown, J. A., Pensabene, V., Markov, D. A., Allwardt, V., Neely, M. D., Shi, M., … Wikswo, J. P. (2015). Recreating blood-brain barrier physiology and structure on chip: A novel neurovascular microfluidic bioreactor. Biomicrofluidics, 9(5), 054124. https://doi.org/10.1063/1.4934713
Mansourpour, M., Mahjub, R., Amini, M., Ostad, S. N., Shamsa, E. S., Rafiee-Tehrani, M., & Dorkoosh, F. A. (2015). Development of acid-resistant alginate/trimethyl chitosan nanoparticles containing cationic β-cyclodextrin polymers for insulin oral delivery. AAPS PharmSciTech, 16(4), 952–962. https://doi.org/10.1208/s12249-014-0282-9
Healy, L. L., Cronin, J. G., & Sheldon, I. M. (2015). Polarized Epithelial Cells Secrete Interleukin 6 Apically in the Bovine Endometrium1. Biology of Reproduction, 92(6), 151. https://doi.org/10.1095/biolreprod.115.127936
Kuehn, D., Majeed, S., Guedj, E., Dulize, R., Baumer, K., Iskandar, A., … Peitsch, M. C. (2015). Impact Assessment of Repeated Exposure of Organotypic 3D Bronchial and Nasal Tissue Culture Models to Whole Cigarette Smoke. Journal of Visualized Experiments, (96), e52325–e52325. https://doi.org/10.3791/52325
Schexnayder, C., & Stratford, R. E. (2015). Genistein and Glyceollin Effects on ABCC2 (MRP2) and ABCG2 (BCRP) in Caco-2 Cells. International Journal of Environmental Research and Public Health, 13(1), ijerph13010017. https://doi.org/10.3390/ijerph13010017
Khan, N., Pantakani, D. V. K., Binder, L., Qasim, M., & Asif, A. R. (2015). Immunosuppressant MPA Modulates Tight Junction through Epigenetic Activation of MLCK/MLC-2 Pathway via p38MAPK. Frontiers in Physiology, 6, 381. https://doi.org/10.3389/fphys.2015.00381
Tian, K. Y., Liu, X. J., Xu, J. D., Deng, L. J., & Wang, G. (2015). Propofol inhibits burn injury-induced hyperpermeability through an apoptotic signal pathway in microvascular endothelial cells. Brazilian Journal of Medical and Biological Research = Revista Brasileira de Pesquisas Medicas e Biologicas, 48(5), 401–407. https://doi.org/10.1590/1414-431X20144107
Schexnayder, C., & Stratford, R. E. (2015). Genistein and Glyceollin Effects on ABCC2 (MRP2) and ABCG2 (BCRP) in Caco-2 Cells. International Journal of Environmental Research and Public Health, 13(1), ijerph13010017. https://doi.org/10.3390/ijerph13010017
Laksitorini, M. D., Kiptoo, P. K., On, N. H., Thliveris, J. A., Miller, D. W., & Siahaan, T. J. (2015). Modulation of intercellular junctions by cyclic-ADT peptides as a method to reversibly increase blood-brain barrier permeability. Journal of Pharmaceutical Sciences, 104(3), 1065–1075. https://doi.org/10.1002/jps.24309
Pacifico, A., Garnet, A., & Reed, K. (2015). Measuring changes in bioavailability of artemisinin. Major Qualifying Projects (All Years). Retrieved from https://digitalcommons.wpi.edu/mqp-all/229
Saeedi, B. J., Kao, D. J., Kitzenberg, D. A., Dobrinskikh, E., Schwisow, K. D., Masterson, J. C., … Glover, L. E. (2015). HIF-dependent regulation of claudin-1 is central to intestinal epithelial tight junction integrity. Molecular Biology of the Cell, 26(12), 2252–2262. https://doi.org/10.1091/mbc.E14-07-1194
Cieza, R. J., Hu, J., Ross, B. N., Sbrana, E., & Torres, A. G. (2015). The IbeA invasin of adherent-invasive Escherichia coli mediates interaction with intestinal epithelia and macrophages. Infection and Immunity, 83(5), 1904–1918. https://doi.org/10.1128/IAI.03003-14
Neilson, L., Mankus, C., Thorne, D., Jackson, G., DeBay, J., & Meredith, C. (2015). Development of an in vitro cytotoxicity model for aerosol exposure using 3D reconstructed human airway tissue; application for assessment of e-cigarette aerosol. Toxicology in Vitro, 29(7), 1952–1962. https://doi.org/10.1016/j.tiv.2015.05.018
Eady, J. J., Wormstone, Y. M., Heaton, S. J., Hilhorst, B., & Elliott, R. M. (2015). Differential effects of basolateral and apical iron supply on iron transport in Caco-2 cells. Genes & Nutrition, 10(3), 463. https://doi.org/10.1007/s12263-015-0463-5
Biswal, M. R., Ahmed, C. M., Ildefonso, C. J., Han, P., Li, H., Jivanji, H., … Lewin, A. S. (2015). Systemic treatment with a 5HT1a agonist induces anti-oxidant protection and preserves the retina from mitochondrial oxidative stress. Experimental Eye Research, 140, 94–105. https://doi.org/10.1016/j.exer.2015.07.022
Hind, W. H., Tufarelli, C., Neophytou, M., Anderson, S. I., England, T. J., & O’Sullivan, S. E. (2015). Endocannabinoids modulate human blood-brain barrier permeability in vitro. British Journal of Pharmacology, 172(12), 3015–3027. https://doi.org/10.1111/bph.13106
Mishra, R., & Singh, S. K. (2014). HIV-1 Tat C phosphorylates VE-cadherin complex and increases human brain microvascular endothelial cell permeability. BMC Neuroscience, 15, 80. https://doi.org/10.1186/1471-2202-15-80
Czupalla, C. J., Liebner, S., & Devraj, K. (2014). In Vitro Models of the Blood–Brain Barrier (pp. 415–437). https://doi.org/10.1007/978-1-4939-0320-7_34
Sun, H., Harris, W. T., Kortyka, S., Kotha, K., Ostmann, A. J., Rezayat, A., … Clancy, J. P. (2014). Tgf-beta downregulation of distinct chloride channels in cystic fibrosis-affected epithelia. PloS One, 9(9), e106842. https://doi.org/10.1371/journal.pone.0106842
Wise, S. K., Laury, A. M., Katz, E. H., Den Beste, K. A., Parkos, C. A., & Nusrat, A. (2014). Interleukin-4 and interleukin-13 compromise the sinonasal epithelial barrier and perturb intercellular junction protein expression. International Forum of Allergy & Rhinology, 4(5), 361–370. https://doi.org/10.1002/alr.21298
Enjoji, S., Ohama, T., & Sato, K. (2014). Regulation of epithelial cell tight junctions by protease-activated receptor 2. The Journal of Veterinary Medical Science, 76(9), 1225–1229. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24881651
Mooren, O. L., Li, J., Nawas, J., & Cooper, J. A. (2014). Endothelial cells use dynamic actin to facilitate lymphocyte transendothelial migration and maintain the monolayer barrier. Molecular Biology of the Cell, 25(25), 4115–4129. https://doi.org/10.1091/mbc.E14-05-0976
Chraïbi, A., & Renauld, S. (2014). PPARγ-induced stimulation of amiloride-sensitive sodium current in renal collecting duct principal cells is serum and insulin dependent. Cellular Physiology and Biochemistry : International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology, 33(3), 581–593. https://doi.org/10.1159/000358636
Schneditz, G., Rentner, J., Roier, S., Pletz, J., Herzog, K. A. T., Bücker, R., … Zechner, E. L. (2014). Enterotoxicity of a nonribosomal peptide causes antibiotic-associated colitis. Proceedings of the National Academy of Sciences of the United States of America, 111(36), 13181–13186. https://doi.org/10.1073/pnas.1403274111
Molenda, N., Urbanova, K., Weiser, N., Kusche-Vihrog, K., Günzel, D., Schillers, H., … Howell, S. (2014). Paracellular Transport through Healthy and Cystic Fibrosis Bronchial Epithelial Cell Lines – Do We Have a Proper Model? PLoS ONE, 9(6), e100621. https://doi.org/10.1371/journal.pone.0100621
Costello, C. M., Hongpeng, J., Shaffiey, S., Yu, J., Jain, N. K., Hackam, D., & March, J. C. (2014). Synthetic small intestinal scaffolds for improved studies of intestinal differentiation. Biotechnology and Bioengineering, 111(6), 1222–1232. https://doi.org/10.1002/bit.25180
Enjoji, S., Ohama, T., & Sato, K. (2014). Regulation of epithelial cell tight junctions by protease-activated receptor 2. The Journal of Veterinary Medical Science, 76(9), 1225–1229. https://doi.org/10.1292/jvms.14-0191
Czupalla, C. J., Liebner, S., & Devraj, K. (2014). In vitro models of the blood-brain barrier. Methods in Molecular Biology (Clifton, N.J.), 1135, 415–437. https://doi.org/10.1007/978-1-4939-0320-7_34
Lei, Y., Stamer, W. D., Wu, J., & Sun, X. (2014). Cell senescence reduced the mechanotransduction sensitivity of porcine angular aqueous plexus cells to elevation of pressure. Investigative Ophthalmology & Visual Science, 55(4), 2324–2328. https://doi.org/10.1167/iovs.13-13317
Yu, J., Li, N., Lin, P., Li, Y., Mao, X., Bao, G., … Zhao, R. (2014). Intestinal transportations of main chemical compositions of polygoni multiflori radix in caco-2 cell model. Evidence-Based Complementary and Alternative Medicine : ECAM, 2014, 483641. https://doi.org/10.1155/2014/483641
Van Itallie, C. M., Tietgens, A. J., Aponte, A., Fredriksson, K., Fanning, A. S., Gucek, M., & Anderson, J. M. (2014). Biotin ligase tagging identifies proteins proximal to E-cadherin, including lipoma preferred partner, a regulator of epithelial cell-cell and cell-substrate adhesion. Journal of Cell Science, 127(Pt 4), 885–895. https://doi.org/10.1242/jcs.140475
Wang, L., Luo, H., Chen, X., Jiang, Y., & Huang, Q. (2014). Functional characterization of S100A8 and S100A9 in altering monolayer permeability of human umbilical endothelial cells. PloS One, 9(3), e90472. https://doi.org/10.1371/journal.pone.0090472
Booth, R., & Kim, H. (2014). Permeability Analysis of Neuroactive Drugs Through a Dynamic Microfluidic In Vitro Blood–Brain Barrier Model. Annals of Biomedical Engineering, 42(12), 2379–2391. https://doi.org/10.1007/s10439-014-1086-5
Nakadate, H., Inuzuka, K., Akanuma, S., Kakuta, A., & Aomura, S. (2014). Effect of amplitude and duration of impulsive pressure on endothelial permeability in in vitro fluid percussion trauma. Biomedical Engineering Online, 13, 44. https://doi.org/10.1186/1475-925X-13-44
Keenan, C. R., Mok, J. S., Harris, T., Xia, Y., Salem, S., & Stewart, A. G. (2014). Bronchial epithelial cells are rendered insensitive to glucocorticoid transactivation by transforming growth factor-β1. Respiratory Research, 15(1), 55. https://doi.org/10.1186/1465-9921-15-55
McIntyre, B. A. S., Alev, C., Mechael, R., Salci, K. R., Lee, J. B., Fiebig-Comyn, A., … Bhatia, M. (2014). Expansive generation of functional airway epithelium from human embryonic stem cells. Stem Cells Translational Medicine, 3(1), 7–17. https://doi.org/10.5966/sctm.2013-0119
Guzman-Aranguez, A., Calvo, P., Ropero, I., & Pintor, J. (2014). In vitro effects of preserved and unpreserved anti-allergic drugs on human corneal epithelial cells. Journal of Ocular Pharmacology and Therapeutics : The Official Journal of the Association for Ocular Pharmacology and Therapeutics, 30(9), 790–798. https://doi.org/10.1089/jop.2014.0030
Li, G., Li, T., Li, Y., Cai, S., Zhang, Z., Zeng, Z., … Chen, Z. (2014). Ulinastatin inhibits oxidant-induced endothelial hyperpermeability and apoptotic signaling. International Journal of Clinical and Experimental Pathology, 7(11), 7342–7350. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/25550770
Sjöqvist, S., Jungebluth, P., Lim, M. L., Haag, J. C., Gustafsson, Y., Lemon, G., … Macchiarini, P. (2014). Experimental orthotopic transplantation of a tissue-engineered oesophagus in rats. Nature Communications, 5, 3562. https://doi.org/10.1038/ncomms4562
Meredith, M. E., Qu, Z.-C., & May, J. M. (2014). Ascorbate reverses high glucose- and RAGE-induced leak of the endothelial permeability barrier. Biochemical and Biophysical Research Communications, 445(1), 30–35. https://doi.org/10.1016/j.bbrc.2014.01.078
Bohara, M., Kambe, Y., Nagayama, T., Tokimura, H., Arita, K., & Miyata, A. (2014). C-type natriuretic peptide modulates permeability of the blood-brain barrier. Journal of Cerebral Blood Flow and Metabolism : Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 34(4), 589–596. https://doi.org/10.1038/jcbfm.2013.234
De Chiara, L., Fagoonee, S., Ranghino, A., Bruno, S., Camussi, G., Tolosano, E., … Altruda, F. (2014). Renal cells from spermatogonial germline stem cells protect against kidney injury. Journal of the American Society of Nephrology : JASN, 25(2), 316–328. https://doi.org/10.1681/ASN.2013040367
Gülseren, İ., Guri, A., & Corredig, M. (2014). Effect of interfacial composition on uptake of curcumin–piperine mixtures in oil in water emulsions by Caco-2 cells. Food & Function, 5(6), 1218. https://doi.org/10.1039/c3fo60554j
Li, Y., Wu, W.-H., Hsu, C.-W., Nguyen, H. V, Tsai, Y.-T., Chan, L., … Tsang, S. H. (2014). Gene therapy in patient-specific stem cell lines and a preclinical model of retinitis pigmentosa with membrane frizzled-related protein defects. Molecular Therapy : The Journal of the American Society of Gene Therapy, 22(9), 1688–1697. https://doi.org/10.1038/mt.2014.100
Al-Ghoul, W. M., Kim, M. S., Fazal, N., Azim, A. C., & Ali, A. (2014). Evidence for simvastatin anti-inflammatory actions based on quantitative analyses of NETosis and other inflammation/oxidation markers. Results in Immunology, 4, 14–22. https://doi.org/10.1016/j.rinim.2014.03.001
Arredondo Zamarripa, D., Díaz-Lezama, N., Meléndez García, R., Chávez Balderas, J., Adán, N., Ledesma-Colunga, M. G., … Thebault, S. (2014). Vasoinhibins regulate the inner and outer blood-retinal barrier and limit retinal oxidative stress. Frontiers in Cellular Neuroscience, 8, 333. https://doi.org/10.3389/fncel.2014.00333
Rizzolo, L. J. (2014). Barrier properties of cultured retinal pigment epithelium. Experimental Eye Research, 126, 16–26. https://doi.org/10.1016/j.exer.2013.12.018
Grover, A., Hirani, A., Pathak, Y., & Sutariya, V. (2014). Brain-targeted delivery of docetaxel by glutathione-coated nanoparticles for brain cancer. AAPS PharmSciTech, 15(6), 1562–1568. https://doi.org/10.1208/s12249-014-0165-0
Meenach, S. A., Anderson, K. W., Hilt, J. Z., McGarry, R. C., Mansour, H. M., Samantha A. Meenach, Kimberly W. Anderson, J. Zach Hilt, Ronald C. McGarry, H. M. M., … Mansour, H. M. (2014, December 20). High-Performing Dry Powder Inhalers of Paclitaxel DPPC/DPPG Lung Surfactant-Mimic Multifunctional Particles in Lung Cancer: Physicochemical Characterization, In Vitro Aerosol Dispersion, and Cellular Studies. https://doi.org/10.1208/s12249-014-0182-z
Fossum, S. L., Mutolo, M. J., Yang, R., Dang, H., O’Neal, W. K., Knowles, M. R., … Harris, A. (2014). Ets homologous factor regulates pathways controlling response to injury in airway epithelial cells. Nucleic Acids Research, 42(22), 13588–13598. https://doi.org/10.1093/nar/gku1146
Roh-Johnson, M., Bravo-Cordero, J. J., Patsialou, A., Sharma, V. P., Guo, P., Liu, H., … Condeelis, J. (2014). Macrophage contact induces RhoA GTPase signaling to trigger tumor cell intravasation. Oncogene, 33(33), 4203–4212. https://doi.org/10.1038/onc.2013.377
Crane, J. K., Broome, J. E., Reddinger, R. M., & Werth, B. B. (2014). Zinc protects against Shiga-toxigenic Escherichia coli by acting on host tissues as well as on bacteria. BMC Microbiology, 14, 145. https://doi.org/10.1186/1471-2180-14-145
Pongkorpsakol, P., Pathomthongtaweechai, N., Srimanote, P., Soodvilai, S., Chatsudthipong, V., & Muanprasat, C. (2014). Inhibition of cAMP-activated intestinal chloride secretion by diclofenac: cellular mechanism and potential application in cholera. PLoS Neglected Tropical Diseases, 8(9), e3119. https://doi.org/10.1371/journal.pntd.0003119
Shimko, M. J., Zaccone, E. J., Thompson, J. A., Schwegler-Berry, D., Kashon, M. L., & Fedan, J. S. (2014). Nerve growth factor reduces amiloride-sensitive Na+ transport in human airway epithelial cells. Physiological Reports, 2(7). https://doi.org/10.14814/phy2.12073
Volpe, D. A., Hamed, S. S., & Zhang, L. K. (2014). Use of Different Parameters and Equations for Calculation of IC50 Values in Efflux Assays: Potential Sources of Variability in IC50 Determination. The AAPS Journal, 16(1), 172–180. https://doi.org/10.1208/s12248-013-9554-7
Costello, C. M., Sorna, R. M., Goh, Y.-L., Cengic, I., Jain, N. K., & March, J. C. (2014). 3-D intestinal scaffolds for evaluating the therapeutic potential of probiotics. Molecular Pharmaceutics, 11(7), 2030–2039. https://doi.org/10.1021/mp5001422
McHugh, K. J., Tao, S. L., & Saint-Geniez, M. (2014). Porous poly(ε-caprolactone) scaffolds for retinal pigment epithelium transplantation. Investigative Ophthalmology & Visual Science, 55(3), 1754–1762. https://doi.org/10.1167/iovs.13-12833
Wise, S. K., Laury, A. M., Katz, E. H., Den Beste, K. A., Parkos, C. A., & Nusrat, A. (2014). Interleukin-4 and interleukin-13 compromise the sinonasal epithelial barrier and perturb intercellular junction protein expression. International Forum of Allergy & Rhinology, 4(5), 361–370. https://doi.org/10.1002/alr.21298
Chimezie, C., Ewing, A. C., Quadri, S. S., Cole, R. B., Boué, S. M., Omari, C. F., … Jr. (2014). Glyceollin transport, metabolism, and effects on p-glycoprotein function in Caco-2 cells. Journal of Medicinal Food, 17(4), 462–471. https://doi.org/10.1089/jmf.2013.0115
Gipson, I. K., Spurr-Michaud, S., Tisdale, A., & Menon, B. B. (2014). Comparison of the transmembrane mucins MUC1 and MUC16 in epithelial barrier function. PloS One, 9(6), e100393. https://doi.org/10.1371/journal.pone.0100393
Reaves, D. K., Fagan-Solis, K. D., Dunphy, K., Oliver, S. D., Scott, D. W., & Fleming, J. M. (2014). The role of lipolysis stimulated lipoprotein receptor in breast cancer and directing breast cancer cell behavior. PloS One, 9(3), e91747. https://doi.org/10.1371/journal.pone.0091747
Uehara, I., Kimura, T., Tanigaki, S., Fukutomi, T., Sakai, K., Shinohara, Y., … Sakurai, H. (2014). Paracellular route is the major urate transport pathway across the blood-placental barrier. Physiological Reports, 2(5). https://doi.org/10.14814/phy2.12013
Couturier, J., Hutchison, A. T., Medina, M. A., Gingaras, C., Urvil, P., Yu, X., … Lewis, D. E. (2014). HIV replication in conjunction with granzyme B production by CCR5+ memory CD4 T cells: Implications for bystander cell and tissue pathologies. Virology, 462–463, 175–188. https://doi.org/10.1016/j.virol.2014.06.008
Eigenmann, D. E., Xue, G., Kim, K. S., Moses, A. V., Hamburger, M., & Oufir, M. (2013). Comparative study of four immortalized human brain capillary endothelial cell lines, hCMEC/D3, hBMEC, TY10, and BB19, and optimization of culture conditions, for an in vitro blood-brain barrier model for drug permeability studies. Fluids and Barriers of the CNS, 10(1), 33. https://doi.org/10.1186/2045-8118-10-33
Ao, M., Sarathy, J., Domingue, J., Alrefai, W. A., & Rao, M. C. (2013). Chenodeoxycholic acid stimulates Cl(-) secretion via cAMP signaling and increases cystic fibrosis transmembrane conductance regulator phosphorylation in T84 cells. American Journal of Physiology. Cell Physiology, 305(4), C447-56. https://doi.org/10.1152/ajpcell.00416.2012
Ghaffarian, R., & Muro, S. (2013). Models and Methods to Evaluate Transport of Drug Delivery Systems Across Cellular Barriers. Journal of Visualized Experiments, (80), e50638–e50638. https://doi.org/10.3791/50638
Chen, L., Zhu, J., Li, Y., Lu, J., Gao, L., Xu, H., … Yang, X. (2013). Enhanced nasal mucosal delivery and immunogenicity of anti-caries DNA vaccine through incorporation of anionic liposomes in chitosan/DNA complexes. PloS One, 8(8), e71953. https://doi.org/10.1371/journal.pone.0071953
Kauffman, A. L., Gyurdieva, A. V., Mabus, J. R., Ferguson, C., Yan, Z., & Hornby, P. J. (2013). Alternative functional in vitro models of human intestinal epithelia. Frontiers in Pharmacology, 4, 79. https://doi.org/10.3389/fphar.2013.00079
Shirasawa, M., Sonoda, S., Terasaki, H., Arimura, N., Otsuka, H., Yamashita, T., … Sakamoto, T. (2013). TNF-α disrupts morphologic and functional barrier properties of polarized retinal pigment epithelium. Experimental Eye Research, 110, 59–69. https://doi.org/10.1016/j.exer.2013.02.012
Close, T. E., Cepinskas, G., Omatsu, T., Rose, K. L., Summers, K., Patterson, E. K., & Fraser, D. D. (2013). Diabetic Ketoacidosis Elicits Systemic Inflammation Associated with Cerebrovascular Endothelial Cell Dysfunction. Microcirculation, 20(6), 534–543. https://doi.org/10.1111/micc.12053
Lei, Y., Stamer, W. D., Wu, J., & Sun, X. (2013). Oxidative stress impact on barrier function of porcine angular aqueous plexus cell monolayers. Investigative Ophthalmology & Visual Science, 54(7), 4827–4835. https://doi.org/10.1167/iovs.12-11435
Guzman-Aranguez, A., Woodward, A. M., Pintor, J., & Argüeso, P. (2012). Targeted disruption of core 1 β1,3-galactosyltransferase (C1galt1) induces apical endocytic trafficking in human corneal keratinocytes. PloS One, 7(5), e36628. https://doi.org/10.1371/journal.pone.0036628
Lippmann, E. S., Azarin, S. M., Kay, J. E., Nessler, R. A., Wilson, H. K., Al-Ahmad, A., … Shusta, E. V. (2012). Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nature Biotechnology, 30(8), 783–791. https://doi.org/10.1038/nbt.2247
Booth, R., & Kim, H. (2012). Characterization of a microfluidic in vitro model of the blood-brain barrier (μBBB). Lab on a Chip, 12(10), 1784. https://doi.org/10.1039/c2lc40094d
Schmedt, T., Chen, Y., Nguyen, T. T., Li, S., Bonanno, J. A., Jurkunas, U. V., … Giasson, C. (2012). Telomerase Immortalization of Human Corneal Endothelial Cells Yields Functional Hexagonal Monolayers. PLoS ONE, 7(12), e51427. https://doi.org/10.1371/journal.pone.0051427
Strengert, M., & Knaus, U. G. (2011). Analysis of Epithelial Barrier Integrity in Polarized Lung Epithelial Cells. In Methods in molecular biology (Clifton, N.J.) (Vol. 763, pp. 195–206). https://doi.org/10.1007/978-1-61779-191-8_13
Wang, Y., & Alexander, J. S. (2011). Analysis of endothelial barrier function in vitro. Methods in Molecular Biology, 763, 253–264. https://doi.org/10.1007/978-1-61779-191-8_17