This website uses cookies to ensure you get the best experience on our website.
Read more
Automated TEER Measurement System - DISCONTINUED
$35,005.00
Prices valid in USA, Canada, and PR only.
Order code
SYS-REMS

Prices valid in USA, Canada, and PR only.
REMS has been discontinued. Upgrade to WPI’s new and advanced version of automated TEER measurement system: EVOM™ Auto with 24 and 96 capability, which has higher measurement range (up to 100 kΩ), wireless connectivity, 2X faster sample analysis with arrays of electrodes for 24 and 96 high throughput screening (HTS) plates. EVOM™ Auto has automated data recording capabilities at specific time intervals, compact footprint to fit into cell culture hood or incubator, and advanced user interface includes ability to create and save sequences with different automated measurement and electrode rinse/disinfection options and use these sequences to run experiments.
Prices valid in USA, Canada, and PR only.
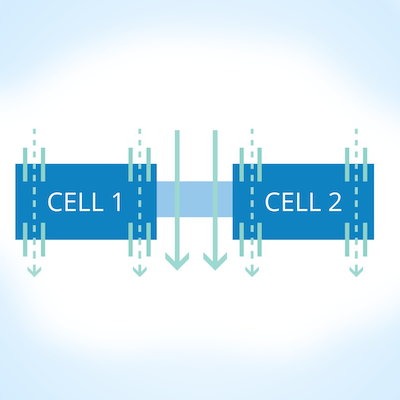
PC-controlled high throughput TEER measurement for epithelial monolayer
The REMS AutoSampler automates measurements of TEER epithelial or endothelial monolayers cultured on HTS well plates. It is a PC-controlled tissue resistance measurement system that offers reproducibility, accuracy, flexibility and ease-of-operation. Automated measurement of tissue resistance in cell culture microplates provides the advantages of speed, precision, decreased opportunity for contamination and the rapid availability of measured resistance data.
Features
- PC controlled positioning
- Data acquisition in LabView
- Manufacturer specific electrodes available for 24 and 96-well HTS plates
- Plate configuration files and sample sequences are user definable
- Two user-defined rinse locations
- Manual mode
NEW application note!
Click here to view the current Data Sheet.
Benefits
- Speed—capable of acquiring TEER data on a 96 well plate in less than five minutes
- Automation reduces the possibility for human error
Applications
- Automated measurement and data logging of TEER for 24 and 96 well HTS culture plates
The REMS AutoSampler automates measurements of TEER epithelial or endothelial monolayers cultured on HTS well plates. It is a PC-controlled tissue resistance measurement system that offers reproducibility, accuracy, flexibility and ease-of-operation. Automated measurement of tissue resistance in cell culture microplates provides the advantages of speed, precision, decreased opportunity for contamination and the rapid availability of measured resistance data.
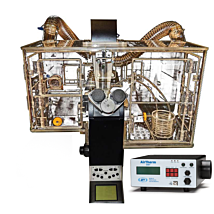
The main components of the REMS AutoSampler include: the robotic sampler that moves the electrode over each well of the microplate, the electrode which is located on the robotic arm, a base plate for the 24 and 96 well tray, a Windows-based data acquisition card, the REMS electrode interface unit and the REMS software to operate the system on a Windows-based computer.
Automate TEER measurements
The REMS AutoSampler automates TEER measurements that would otherwise be performed manually with WPI’s EVOM2™ Epithelial Voltohmmeter. Automated tissue resistance measurements up to 20 kΩ can be performed on 24 or 96 well HTS microplates. See WPI’s website for manufacturer plate compatibility.
Precisely and reproducibly positions electrode
The REMS AutoSampler will automatically measure and record tissue resistance from a user-specified matrix of culture wells on the microplate. According to the specified sequence, the robotic arm moves over the identified wells taking TEER measurements. By means of an x-y-z locating system, the electrode is positioned precisely into the well. The ability of the REMS AutoSampler to reproducibly position the electrode contributes to consistent TEER measurements. TEER data is incrementally stored as the electrode moves from one well to the next.
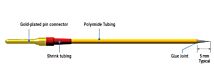
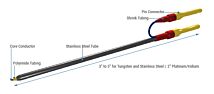
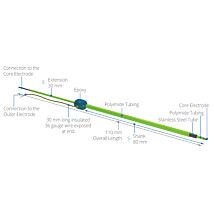
Compact electrode pair
The use of AC current to measure resistance provides several advantages over DC current, including:
- Absence of offset voltages on measurements
- There is a zero net current being passed through the membrane and, therefore, it is not adversely affected by a current charge
- No electrochemical deposition of electrode metal.
Rinse and calibration check stations
The REMS AutoSampler also features two rinse stations. If occasional rinsing of the REMS electrode is required it may be sent to a rinse station by pressing the rinse station button on the menu bar.
Compatible Plates
The following plates can be used with the REMS system:
| REMS-24 | REMS-24M | REMS-96 | REMS-96 | REMS-96C | |
| Corning HTS Transwell | Corning Falcon HTS MultiWell | Millicell-24 | Millipore HTS96 Multiwell | Corning Falcon | Corning HTS Transwell |
| 3378 3379 3396 3397 3398 3399 |
351181 351183 351185 354803 354804 |
PSHT010R1 |
PSHT004R1 |
351131 351161 351162 351163 351164 353938 |
3380 3392 3381 3391 3385 3386 3387 3388 3374 3384 |
Sample Plates
 Click to enlarge. |
 Click to enlarge. |
 Click to enlarge. |
 Click to enlarge. |
 Click to enlarge. |
|
This is a Corning #3378 24-well plate that may be used with the REMS-24 or the STX100C. The Corning Falcon 24-well plate is virtually identical to this one. For use with REMS-24 or STX100F. |
This is a Corning Falcon 96-well plate that may be used with the REMS-96. |
This is a Millipore Multiscreen CaCo2 that may be used with the REMS-96 or the STX100M. | This is a Corning 3391 HTS Traswell 96 plate that may be used with the REMS-96C or the STX100C96. | This is a Millicell-24 Cell Culture Insert Plate that may be used with the REMS-24M |
| SKU | SYS-REMS |
|---|
Upsell Products
-
Replacement REMS HTS Electrode
As low as $6,000.00
REMS Installation Instructions
Video
The video below demonstrates the REMS robotic automated TEER measurement system and discusses the recent updates made to the software.
| MEMBRANE RESISTANCE RANGE | 0 to 2000Ω and 0 to 20kΩ |
| AC SQUARE WAVE CURRENT | +/- 20μA @ 12.5 Hz |
| ELECTRODE POSITIONING | Resolution in X, Y and Z: +/- 1mm |
| ELECTRODE PERFORMANCE | Repeatability in X, Y and Z: +/- 0.25mm |
| ELECTRODE ARM SPEED | X- and Y-axis: 250mm/sec Z-axis: 247.3mm/sec |
| TYPICAL MEASUREMENT TIME 24-WELL | 1 min, 10 sec |
| SCAN PATTERN | Preset or user-defined |
| LINE VOLTAGE | User specified: 100/120 V or 220/240 V |
| DIMENSIONS | 53.5 x 43.7 x 37.1 cm (21.09 x 17.19 x 14.63 in.) |
| WEIGHT | 24 kg (52 lb) |
The system includes robot sampler, data acquisition board, computer, display, keyboard, mouse, software for Windows 7 or Windows 10, and electrode for either 24-well plate (Corning Costar HTS Transwell-24 or Falcon HTS Multiwell) or 96-well plate (Millipore Multiscreen CaCo or Corning 96). Please specify which plate when ordering.
REMS is supplied with a desktop computer. The 2017 computer shipped with the REMS system is an Intel I5, 3.2 GHz quad core, 4 Gb RAM, 500Gb hard drive, Windows 10 with a monitor, keyboard and mouse.