This website uses cookies to ensure you get the best experience on our website.
Read more
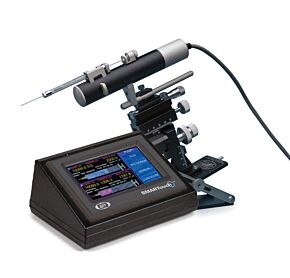
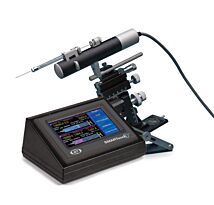
UMP3 UltraMicroPump
As low as
$2,249.00
Only %1 left
Prices valid in USA, Canada, and PR only.
Order code

Prices valid in USA, Canada, and PR only.
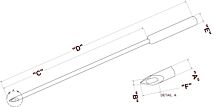
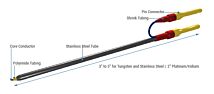
With its digital controller, UltraMicroPump III can repeatably inject as little as 25nL. Syringes may be filled externally and then inserted into the pump or front-filled while mounted on the pump.
Prices valid in USA, Canada, and PR only.
Features
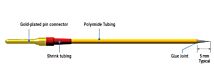
- Versatile UMP3 micro syringe pump injector that uses micro syringes to deliver microliter and nanoliter volumes
- Syringes are easily installed, just snap the barrel into the clamps
- Threaded shaft drives syringe plunger allowing smooth and accurate movement
Click here to view the current Data Sheet.
Options
| Order code | Description | Micro4 Controller |
|
Single UMP3 Pump Head ONLY |
No |
|
|
UMP3-3 |
Three UMP3 Pumps with Controller |
Yes |
|
UMP3-4 |
Four UMP3 Pumps with Controller |
Yes |
|
SYS-MICRO4 |
4-Channel Controller ONLY |
Yes |
Note : UMP3 microinjection syringe pump includes the pump head only. For controller information, see Micro4.
Nanoliter volumes
With its digital controller, UltraMicroPump III can dispense as little as 600 picoliters per incremental advance of the syringe piston (using a 0.5μL syringe). Syringes may be filled externally and then inserted into the pump or filled while mounted in the pump. Fluids injected or withdrawn are held entirely within the micro syringe to maintain a low fluid dead volume in the micropump.
Positioning
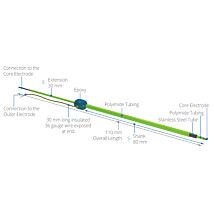
For positioning, the UltraMicroPump III may be attached to any of several WPI micropositioners such as the M3301 (manual), DC3001 (motorized), or any manual stereotaxic manipulator.
Smart Controller
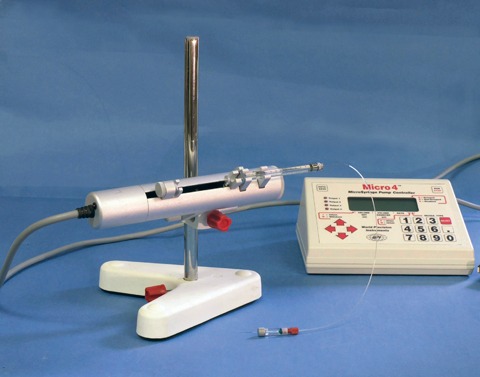
An Integral component in the UMPIII microinjection syringe pump system is a microprocessor-based controller, MICRO4, which provides an "intelligent" and easy-to-use interface to up to four syringe pumps. Operating parameters are set with the membrane keypad and LCD display. From the keypad you can select the following functions:
- set the microliter syringe pump to infusion or withdrawal mode
- enter the volume to be infused or withdrawn, rate of delivery, and syringe type
- synchronize the starting and stopping of any combination of syringe pumps
User parameters can be stored in the controller's "non-volatile" memory for instant recall when the unit is powered on. An optional footswitch can be plugged into a connector on the rear of the controller for "hands free" start/stop operation.
Computer Control
An RS-232 port on the rear of the controller can be used to connect it to a computer for use with computer control programs.
The 503207 kit is ideal for working with an UMP3 microinjection syringe pump as shown below.
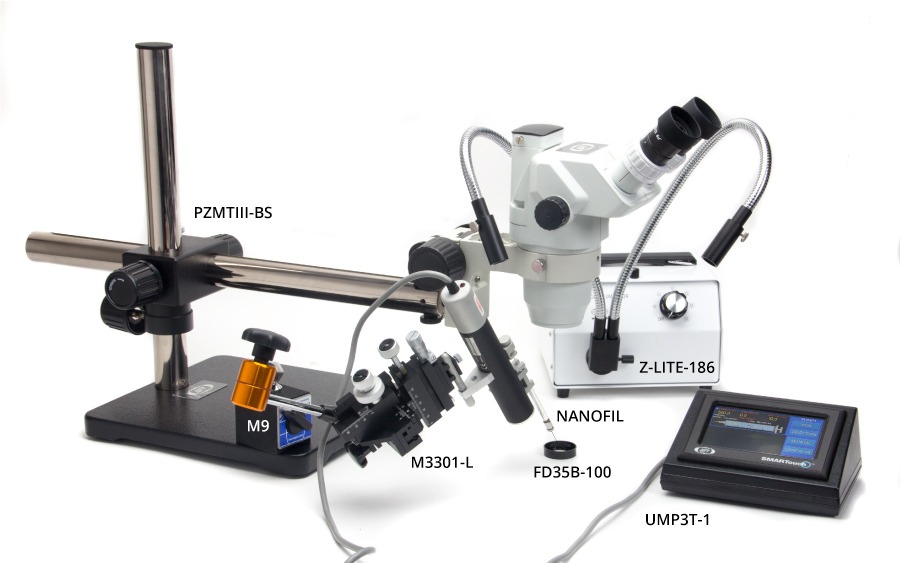
The following images show various setups for microinjection. Keep in mind that parts are interchangeable. For example:
- M10 or the M9 magnetic base could be used.
- PZMIV microscope could be used instead of the PZMIII.
- M330l or the KITE micromanipulators can be used, and these micromanipulators can be placed on either side. (Keep in mind, though, If you wanted to use a KITE on the right side of the setup below, you would order a KITE-R (right hand), or if you want an M3301 on the left side, you would order an M3301-L.)
- 5479 or 5052 magnetic bases are virtually interchangeable.
- One or two Nanoliters, one or two UMPIII systems, or one Nanoliter and one UMPIII may be used, as desired.
ONE NANOLITER/ONE UMP3-1

ONE UMP3-1
| SKU | VAR-3735 |
|---|
Upsell Products
- As low as $115.00
- $150.00
- As low as $158.00
- As low as $761.00
Syringe Volume Calculation Spreadsheet - Use this .XLS spreadsheet to calculate the volume of your syringe when you are using an UMP3, DMP, MMP or PV820/PV830.
Normal Mode
| Total # of Steps | 20,000(63mm travel) |
| Minimum Dispensing Volume | 25nL |
| Linear Motion per Step | 3.175μm |
| Weight | 325g (11.5 oz.) |
| Mounting Rod Diameters | 7.9mm (0.31 in.) |
| Controller Power Requirements | 2A, 12VDC |
| Dimensions | Ø32mm x 190mm (1.3 in. x 7.5 in.) |
Microstepping Mode
Precision is increased eight fold.
Zhou, Z., Luther, N., Singh, R., Boockvar, J. A., Souweidane, M. M., & Greenfield, J. P. (2017). Glioblastoma spheroids produce infiltrative gliomas in the rat brainstem. Child’s Nervous System, 1–10. http://doi.org/10.1007/s00381-017-3344-y
Ye, H.-L., Li, D.-R., Yang, J.-S., Chen, D.-F., De Vos, S., Vuylsteke, M., … Yang, W.-J. (2017). Molecular characterization and functional analyses of a diapause hormone receptor-like gene in parthenogenetic Artemia. Peptides. http://doi.org/10.1016/j.peptides.2017.01.008
Wofford, K. L., Harris, J. P., Browne, K. D., Brown, D. P., Grovola, M. R., Mietus, C. J., … Cullen, D. K. (2017). Rapid neuroinflammatory response localized to injured neurons after diffuse traumatic brain injury in swine. Experimental Neurology, 290, 85–94. http://doi.org/10.1016/j.expneurol.2017.01.004
Qi, Y., Purtell, L., Fu, M., Zhang, L., Zolotukhin, S., Campbell, L., & Herzog, H. (2017). Hypothalamus specific re-introduction of Snord116 into otherwise Snord116 deficient mice increased energy expenditure. Journal of Neuroendocrinology. http://doi.org/10.1111/jne.12457
Mosberger, A. C., Miehlbradt, J. C., Bjelopoljak, N., Schneider, M. P., Wahl, A.-S., Ineichen, B. V., … Schwab, M. E. (2017). Axotomized Corticospinal Neurons Increase Supra-Lesional Innervation and Remain Crucial for Skilled Reaching after Bilateral Pyramidotomy. Cerebral Cortex, 137, 1716–1732. http://doi.org/10.1093/cercor/bhw405
Job, M. O., & Kuhar, M. J. (2017). CART peptide in the nucleus accumbens regulates psychostimulants: Correlations between psychostimulant and CART peptide effects. Neuroscience, 348, 135–142. http://doi.org/10.1016/j.neuroscience.2017.02.012
Eleftheriadou, I., Dieringer, M., Poh, X. Y., Sanchez-Garrido, J., Gao, Y., Sgourou, A., … Mazarakis, N. D. (2017). Selective transduction of astrocytic and neuronal CNS subpopulations by lentiviral vectors pseudotyped with Chikungunya virus envelope. Biomaterials, 123, 1–14. http://doi.org/10.1016/j.biomaterials.2017.01.023
Augestad, I. L., Nyman, A. K. G., Costa, A. I., Barnett, S. C., Sandvig, A., Håberg, A. K., & Sandvig, I. (2017). Effects of Neural Stem Cell and Olfactory Ensheathing Cell Co-transplants on Tissue Remodelling After Transient Focal Cerebral Ischemia in the Adult Rat. Neurochemical Research, 1–11. http://doi.org/10.1007/s11064-016-2098-3
Lin, P., Fang, Z., Liu, J., & Lee, J. H. (2016). Optogenetic Functional MRI. Journal of Visualized Experiments, (110), e53346–e53346. http://doi.org/10.3791/53346
Vacca, O., El Mathari, B., Darche, M., Sahel, J.-A., Rendon, A., & Dalkara, D. (2015). Using Adeno-associated Virus as a Tool to Study Retinal Barriers in Disease. Journal of Visualized Experiments, (98), e52451–e52451. http://doi.org/10.3791/52451
Lai, J., Legault, M.-A., Thomas, S., & Casanova, C. (2015). Simultaneous Electrophysiological Recording and Micro-injections of Inhibitory Agents in the Rodent Brain. Journal of Visualized Experiments, (101), e52271–e52271. http://doi.org/10.3791/52271
Robinson, S., & Adelman, J. S. (2015). A Method for Remotely Silencing Neural Activity in Rodents During Discrete Phases of Learning. Journal of Visualized Experiments, (100), e52859–e52859. http://doi.org/10.3791/52859
Platt, R. J., Chen, S., Zhou, Y., Yim, M. J., Swiech, L., Kempton, H. R., … Zhang, F. (2014). CRISPR-Cas9 Knockin Mice for Genome Editing and Cancer Modeling. Cell, 159(2), 440–55. http://doi.org/10.1016/j.cell.2014.09.014
Pierce, A. M., & Keating, A. K. (2014). Creating Anatomically Accurate and Reproducible Intracranial Xenografts of Human Brain Tumors. Journal of Visualized Experiments, (91), e52017–e52017. http://doi.org/10.3791/52017
Paveliev, M., Kislin, M., Molotkov, D., Yuryev, M., Rauvala, H., & Khiroug, L. (2014). Acute Brain Trauma in Mice Followed By Longitudinal Two-photon Imaging. Journal of Visualized Experiments : JoVE, (April), 1–8. http://doi.org/10.3791/51559
Nakamura, S., Baratta, M. V., & Cooper, D. C. (2013). A Method for High Fidelity Optogenetic Control of Individual Pyramidal Neurons <em>In vivo</em> Journal of Visualized Experiments, (79), e50291–e50291. http://doi.org/10.3791/50291
Inquimbert, P., Moll, M., Kohno, T., & Scholz, J. (2013). Stereotaxic Injection of a Viral Vector for Conditional Gene Manipulation in the Mouse Spinal Cord. Journal of Visualized Experiments, (73), e50313–e50313. http://doi.org/10.3791/50313
Hewing, N. J., Weskamp, G., Vermaat, J., Farage, E., Glomski, K., Swendeman, S., … Blobel, C. P. (2013). Intravitreal injection of TIMP3 or the EGFR inhibitor erlotinib offers protection from oxygen-induced retinopathy in mice. Investigative Ophthalmology & Visual Science, 54(1), 864–70. http://doi.org/10.1167/iovs.12-10954
Salt, A. N., Hartsock, J. J., Gill, R. M., Piu, F., & Plontke, S. K. (2012). Perilymph Pharmacokinetics of Markers and Dexamethasone Applied and Sampled at the Lateral Semi-Circular Canal. Journal of the Association for Research in Otolaryngology, 13(6), 771–783. http://doi.org/10.1007/s10162-012-0347-y
Nickerson, J. M., Goodman, P., Chrenek, M. A., Bernal, C. J., Berglin, L., Redmond, T. M., & Boatright, J. H. (2012). Subretinal delivery and electroporation in pigmented and nonpigmented adult mouse eyes. Methods in Molecular Biology (Clifton, N.J.), 884, 53–69. http://doi.org/10.1007/978-1-61779-848-1_4
Beier, K., & Cepko, C. (2012). Viral Tracing of Genetically Defined Neural Circuitry. Journal of Visualized Experiments, (68), e4253–e4253. http://doi.org/10.3791/4253
Goel, M., Sienkiewicz, A. E., Picciani, R., Wang, J., Lee, R. K., & Bhattacharya, S. K. (2012). Cochlin, intraocular pressure regulation and mechanosensing. PloS One, 7(4), e34309. http://doi.org/10.1371/journal.pone.0034309
Abdelwahab, M. G., Sankar, T., Preul, M. C., & Scheck, A. C. (2011). Intracranial Implantation with Subsequent 3D <em>In Vivo</em> Bioluminescent Imaging of Murine Gliomas. Journal of Visualized Experiments, (57), e3403–e3403. http://doi.org/10.3791/3403
Lowery, R. L., & Majewska, A. K. (2010). Intracranial Injection of Adeno-associated Viral Vectors. Journal of Visualized Experiments, (45), e2140–e2140. http://doi.org/10.3791/2140
Kinkel, M. D., Eames, S. C., Philipson, L. H., & Prince, V. E. (2010). Intraperitoneal injection into adult zebrafish. Journal of Visualized Experiments : JoVE, (42), e2126. http://doi.org/10.3791/2126
Molotkov, D. A., Yukin, A. Y., Afzalov, R. A., & Khiroug, L. S. (2010). Gene Delivery to Postnatal Rat Brain by Non-ventricular Plasmid Injection and Electroporation. Journal of Visualized Experiments, (43), e2244–e2244. http://doi.org/10.3791/2244
Marker, D. F., Tremblay, M.-E., Lu, S.-M., Majewska, A. K., & Gelbard, H. A. (2010). A Thin-skull Window Technique for Chronic Two-photon <em>In vivo</em> Imaging of Murine Microglia in Models of Neuroinflammation. Journal of Visualized Experiments, (43), e2059–e2059. http://doi.org/10.3791/2059
Eames, S. C., Philipson, L. H., Prince, V. E., & Kinkel, M. D. (2010). Blood sugar measurement in zebrafish reveals dynamics of glucose homeostasis. Zebrafish, 7(2), 205–13. http://doi.org/10.1089/zeb.2009.0640
Jasnow, A. M., Rainnie, D. G., Maguschak, K. A., Chhatwal, J. P., & Ressler, K. J. (2009). Construction of Cell-Type Specific Promoter Lentiviruses for Optically Guiding Electrophysiological Recordings and for Targeted Gene Delivery (pp. 199–213). http://doi.org/10.1007/978-1-59745-559-6_13
Christiana J. Johnson, Lennart Berglin, Micah A. Chrenek, T.M. Redmond, Jeffrey H. Boatright, J. M. N. (2008). Technical Brief: Subretinal injection and electroporation into adult mouse eyes. Molecular Vission, 14, 2211–2226. Retrieved from http://www.molvis.org/molvis/v14/a259/
Takayama, K., Torashima, T., Horiuchi, H., & Hirai, H. (2008). Purkinje-cell-preferential transduction by lentiviral vectors with the murine stem cell virus promoter. Neuroscience Letters (Vol. 443).
Torashima, T., Yamada, N., Itoh, M., Yamamoto, A., & Hirai, H. (2006). Exposure of lentiviral vectors to subneutral pH shifts the tropism from Purkinje cell to Bergmann glia. European Journal of Neuroscience, 24(2), 371–380. http://doi.org/10.1111/j.1460-9568.2006.04927.x
Torashima, T., Okoyama, S., Nishizaki, T., & Hirai, H. (2006). In vivo transduction of murine cerebellar Purkinje cells by HIV-derived lentiviral vectors. Brain Research, 1082(1), 11–22. http://doi.org/10.1016/j.brainres.2006.01.104
Dancause, N., Barbay, S., Frost, S. B., Plautz, E. J., Chen, D., Zoubina, E. V, … Nudo, R. J. (n.d.) (2005). Development/Plasticity/Repair Extensive Cortical Rewiring after Brain Injury. https://doi.org/10.1523/JNEUROSCI.3256-05.2005
Cherezov, V., Peddi, A., Muthusubramaniam, L., Zheng, Y. F., & Caffrey, M. (2004). A robotic system for crystallizing membrane and soluble proteins in lipidic mesophases. Acta Crystallographica Section D Biological Crystallography, 60(10), 1795–1807. http://doi.org/10.1107/S0907444904019109
Bernd, A. S., Aihara, M., Lindsey, J. D., & Weinreb, R. N. (2004). Influence of Molecular Weight on Intracameral Dextran Movement to the Posterior Segment of the Mouse Eye. Investigative Opthalmology & Visual Science, 45(2), 480. http://doi.org/10.1167/iovs.03-0462
Shawgo, R. S. (2004). In vivo activation and biocompatibility of a MEMS microreservoir drug delivery device. Retrieved November 16, 2016, from http://citeweb.info/20041104095
Sturbaum, G. D., Reed, C., Hoover, P. J., Jost, B. H., Marshall, M. M., & Sterling, C. R. (2001). Species-Specific, Nested PCR-Restriction Fragment Length Polymorphism Detection of Single Cryptosporidium parvum Oocysts. APPLIED AND ENVIRONMENTAL MICROBIOLOGY, 67(6), 2665–2668. https://www.ncbi.nlm.nih.gov/pubmed/11375178
Nelson, B. P., Grimsrud, T. E., Liles, M. R., Goodman, R. M., & Corn, R. M. (n.d.) (2001). Surface Plasmon Resonance Imaging Measurements of DNA and RNA Hybridization Adsorption onto DNA Microarrays. https://doi.org/10.1021/ac0010431