This website uses cookies to ensure you get the best experience on our website.
Read more
FREE RADICAL ANALYZER 4CHANNEL
$10,186.00
Prices valid in USA, Canada, and PR only.
Order code
TBR4100

Prices valid in USA, Canada, and PR only.
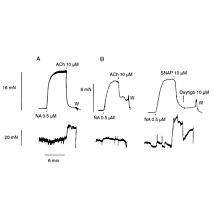
Real-time detection and measurement of a variety of redox-reactive species is fast and easy using the electrochemical (amperometric) detection principle employed in the TBR4100. This optically isolated four-channel free radical analyzer has ultra low noise and independently operated channels.
To learn more about our warranty options, click here.
Prices valid in USA, Canada, and PR only.
Fast, reliable, real-time detection – measure redox-reactive species
Features
- Real-time detection using electrochemical microsensors
- Integrated system includes one temperature sensor, your choice of two additional sensors and a start-up kit
- Current measurement range from 300 fA to 10 µA (four ranges) permits wide dynamic range for detection
- Wide bandwidth allows recording of fast events
- Measure carbon monoxide from 10 nM to 10 µM
- Measure nitric oxide from < 0.3 nM to 100 µM
- Measure hydrogen peroxide < 10 nM to 100 mM
- Measure hydrogen sulfide
- Measure glucose
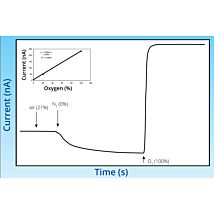
- Measure oxygen from 0.1% to 100%
- Isolated architecture allows Lab-Trax interface to simultaneously measure free radical and independent analog data (for example, ECG, BP, etc.) on any channel
- Four channel free radical detection
Benefits
- Measure up to four different species and temperature in the same preparation or simultaneous measurement in four different preparations
- Lab-Trax data acquisition system is flexible
- Premium Warranty Available
Applications
- Free radical detection (NO, H2O2, H2S, CO, O2 and glucose)
Click here to view the current Data Sheet.
Measure multiple species simultaneously
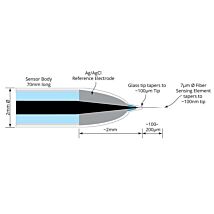
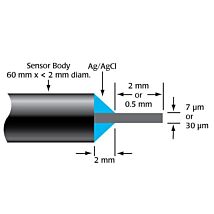
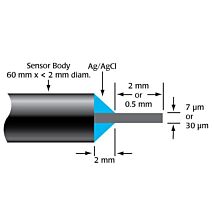
The TBR is designed for use with WPI’s wide range of nitric oxide, hydrogen peroxide, hydrogen sulfide and oxygen sensors. The TBR4100 can measure four different species simultaneously in the same preparation. Simply plug a sensor into the input channel on the front panel and select the current range. Poise voltage can be selected from a range of values tuned for optimal response from WPI sensors. An independent output for real-time monitoring of temperature is also included.
Lab-Trax data acquisition system is flexible
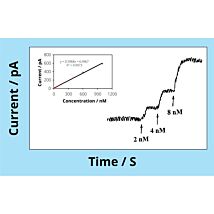
The TBR1025 analyzer utilizes PC-based data acquisition via our Lab-Trax interface. Data traces are displayed and recorded in real-time. The LabScribe software (formerly called DataTrax) comes pre-configured for single or multiple electrode recording; filters, gains, and smoothing are all set for optimal results. Data can be viewed making adjustments to smoothing and filter settings without affecting the original stored raw data. Electrode calibration from multiple concentration readings can be input into the software's Multipoint Calibration utility quickly provides a plot and slope calculation for electrode sensitivity determination.
Alternately, the Lab-Trax data interface can be used for providing simultaneous acquisition of Free Radical data along with other physiological data (ECG, HR, BP, etc.) as each of the four input channels has its own independent input, filters and 24-bit converter.
Start-up systems
TBR4100-416 includes TBR4100 analyzer and power cord, Lab-Trax-4/16 data logger system and USB cable, 4 BNC cables, 3 electrode adapter cables, 1 temperature probe, 2 sensors of your choice, and sensor start-up kit(s), if applicable.
| SKU | TBR4100 |
|---|
Upsell Products
-
ISO-NOPF Flexible Nitric Oxide Sensor
As low as $1,375.00 -
Hydrogen Peroxide Microsensors
As low as $1,525.00
Manuals
TBR Instruction Manual
LabScribe 3 Instruction Manual
Sample Files – ZIP file including hardware and software manuals, NO Demo recording, concentration spreadsheet examples. (Templates_LS3.zip)
Video
The video below shows how to calibrate your oxygen sensor (6 minutes).
| Power | 100 ~ 240 VAC, 50-60 Hz, | ||||||||||||||||||||
| Operating Temperature (ambient) | 0 - 50°C (32 - 122°F) | ||||||||||||||||||||
| Operating Humidity (ambient) | 15 - 70% RH non-condensing | ||||||||||||||||||||
| Warm up Time | < 5 min. | ||||||||||||||||||||
| Dimensions | 135 X 419 X 217 mm (5.25" X 16.5" X 8.16") | ||||||||||||||||||||
| Weight | 1.35 kg (3 lb.) | ||||||||||||||||||||
| Display Functions | 18 mm (0.7") LCD readout, 4.5 digit Polarization Voltage (mV) Current input (nA, µA) | ||||||||||||||||||||
| Controls | Power (on/off) Current Input Range Polarization Voltage |
||||||||||||||||||||
| Analog Output Range | ±10 V (continuous) | ||||||||||||||||||||
| Analog Output Impedance | 10 KΩ | ||||||||||||||||||||
| Channel to Channel Isolation | >10 GΩ | ||||||||||||||||||||
| Channel to Output Isolation | >10 GΩ | ||||||||||||||||||||
| Power Supply to AC Line Isolation | >100 MΩ | ||||||||||||||||||||
| Analog Output Drift | < 10 pA/hr. | ||||||||||||||||||||
| Temperature Input: Number of Channels | 1 | ||||||||||||||||||||
| Temperature Input: Sensing Element | Platinum RTD, 1000 Ω | ||||||||||||||||||||
| Temperature Input: Range | 0-100°C | ||||||||||||||||||||
| Temperature Input: Accuracy | ± 1°C | ||||||||||||||||||||
| Temperature Input: Resolution | 0.1°C | ||||||||||||||||||||
| Temperature Input: Analog Output | 31.25 mV/°C (continuous) | ||||||||||||||||||||
| Amperometric Input: Number of Amperometric Channels | 4 | ||||||||||||||||||||
| Amperometric Input: Signal Bandwidth | 0-3 Hz | ||||||||||||||||||||
| Amperometric Input: Polarization Voltage (selectable via rotary switch) Nitric Oxide | 865 mV | ||||||||||||||||||||
| Amperometric Input: Polarization Voltage (selectable via rotary switch) Hydrogen Sulfide | 150 mV | ||||||||||||||||||||
| Amperometric Input: Polarization Voltage (selectable via rotary switch) Hydrogen Peroxide | 450 mV | ||||||||||||||||||||
| Amperometric Input: Polarization Voltage (selectable via rotary switch) Glucose | 600 mV | ||||||||||||||||||||
| Amperometric Input: Polarization Voltage (selectable via rotary switch) Oxygen | 700 mV | ||||||||||||||||||||
| Amperometric Input: Polarization Voltage (selectable via rotary switch) ADJ (user adjustable) | ± 2500 mV | ||||||||||||||||||||
| Polarization Voltage Accuracy | ± 5 mV | ||||||||||||||||||||
| Polarization Voltage Display Resolution | ± 1mV | ||||||||||||||||||||
| Current measurement Performance: |
|
||||||||||||||||||||
| Notes: | *Instrument performance is measured as the (max-min) over 20 seconds period with open input. Typical values are given at 3 Hz and 0.3 Hz bandwidth. | ||||||||||||||||||||
| Typical sensor performance with TBR4100: ISO-NOPF100 noise | 0.2 nM NO (< 2pA **) | ||||||||||||||||||||
| Notes: | **Sensor noise is measured as the (max-min) over a 20 seconds period with the sensor immersed in 0.1 M CuCl2 solution. |