This website uses cookies to ensure you get the best experience on our website.
Read more
KIT, SILICON SEALANT
$137.00
Prices valid in USA, Canada, and PR only.
Order code
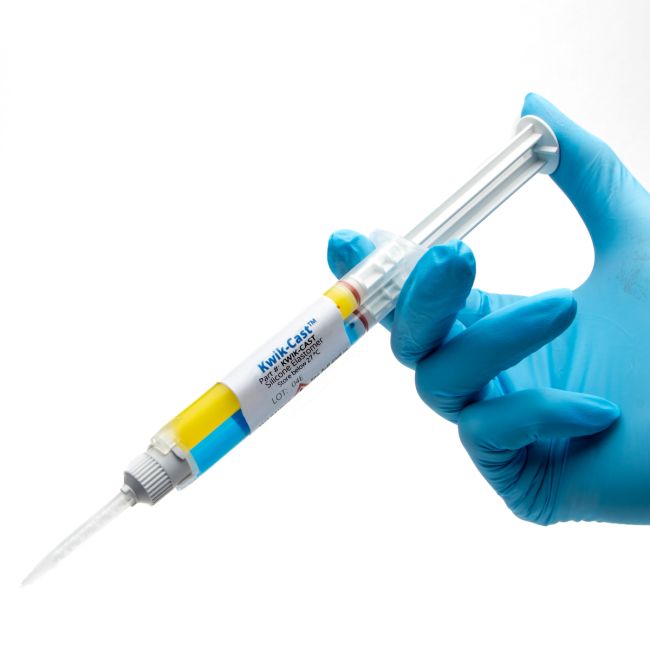
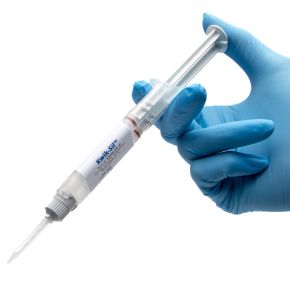
KWIK-CAST

Prices valid in USA, Canada, and PR only.
The properties of this bio-compatible surgical adhesive make it exceptionally useful for neuroscience applications, peripheral nerve studies and similar biomedical applications. These silicone elastomers also eliminate the mess and time involved in pre-mixing other commonly used formulations (such as Wacker SilGel®and Sylgard®). Each silicone elastomer is packaged in a double barrel syringe and is automatically mixed when pressed out of the mixer tip provided. It can then be applied directly to the tissue without further mixing. The curing time of these silicone adhesives is short, reliable and reproducible. This eliminates potential costly guesswork when using other tissue adhesives. The curing process does not produce any heat, which can cause tissue damage. WPI's silicone elastomers are much less toxic than dental silicone, because they contain no surfactant additives.
A sterile version of this product is available as KWIK-CAST-S.
*New and Improved with Extended Shelf Life
Prices valid in USA, Canada, and PR only.
Silicone sealant ideal for neuroscience applications, nerve studies and more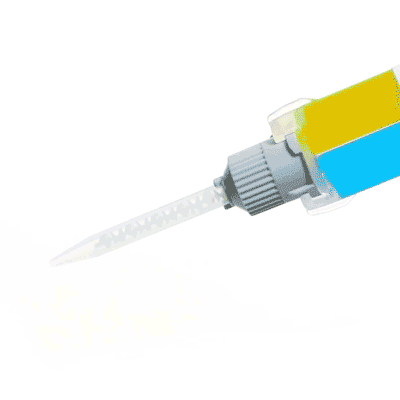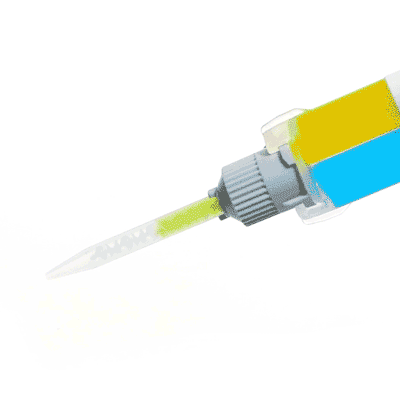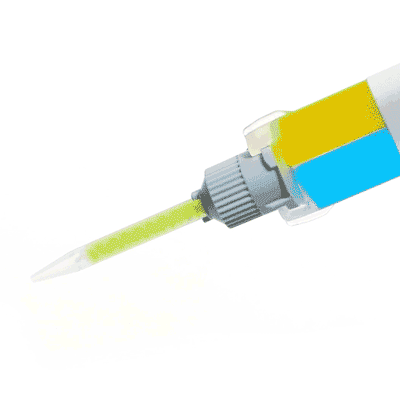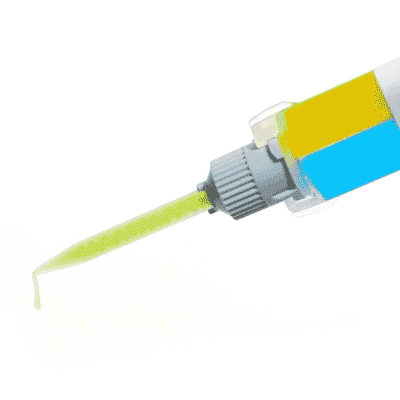
- Biocompatible adhesive to seal live tissues
- Embed peripheral nerves with electrodes
- Pre-mixing tips simplify use (Includes 10 mixing tips)
- Medium strength adhesion
- Low toxicity
- Rapid curing silicone adhesive, cure on contact
- Cures without producing heat
- The kit comes with (2) cartridges of 2 part silicone
- Kwik-Sil dries clear; Kwik-Cast dries green
- Kwik-Sil dries a little faster than Kwik-Cast
Low Toxicity Silicone Adhesive
See for yourself why KWIK-CAST silicone adhesive is so popular for life science applications. When you are looking for a low toxicity adhesive with some elasticity and good moisture resistance, a silicone adhesive is the option of choice. When used with living tissue, an adhesive must be:
- Non-toxic
- Fast Curing
- Able to adhere to organic and inorganic surfaces
Kwik-Sil™ and Kwik-Cast™ silicone adhesives have very low toxicity before, during and after curing. The by-product of curing is a small amount of hydrogen gas, which is much less toxic to cells than acetic acid or alcohol from traditional RTV silicones.
Kwik-Sil™ and Kwik-Cast™ have a curing speed that is hundreds of times faster than traditional RTV silicones. A curing time of a few minutes at room temperature is especially useful for encapsulation of live tissue or implanting into a live animal.
Kwik-Sil™ and Kwik-Cast™ surgical adhesives are not sensitive to contamination from animal tissue, unlike many vinyl-based silicones in which the platinum complex catalysts which are easily poisoned by contamination from amines and animal tissue.
Kwik-Sil™ vs Kwik-Cast™
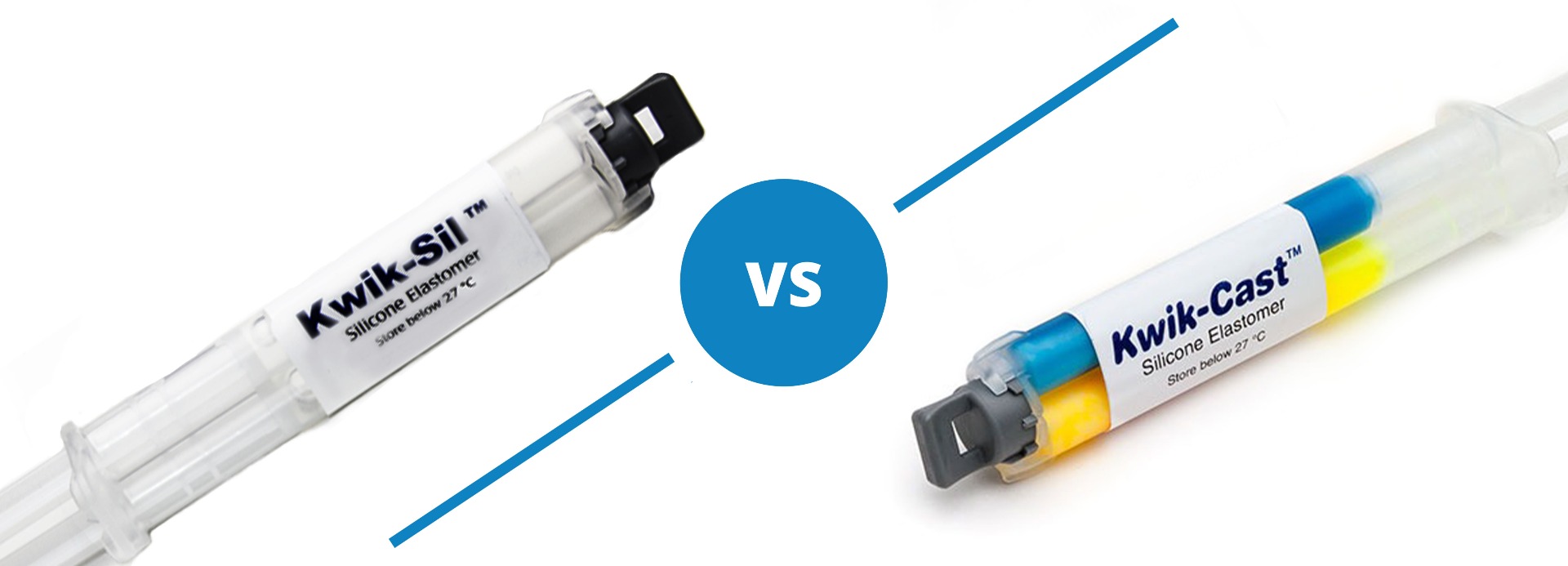
Both Kwik-Sil and Kwik-Cast are silicone adhesives, but they are a little different.
Kwik-Sil™ is a translucent, medium-viscosity silicone adhesive, developed for chronic peripheral nerve studies such as anterograde tracing with fluorescent indicators or electrode recording. Good adhesion and mechanical properties (tear strength and elongation) allow days of study without breaking of the bonding. Curing speed is very reproducible.
Kwik-Cast™ is a very low viscosity silicone sealant developed to embed peripheral nerves with electrodes for acute multi-fiber recordings. It flows easily, filling the small spaces around the nerve and leaving no channels through which peritoneal fluid can travel and thus short the nerve/electrode contact. Equally important is the ability of the material to flow into itself and create one continuous mass from underneath the nerve all the way to the top of the nerve/electrode contact to ensure long-term recording stability. Kwik-Cast is color-coded to make the mixing foolproof. The catalyst is yellow and the base is blue. When uniformly mixed, it is green. Kwik-Cast can be applied and cured underneath mineral oil. After recording, electrodes are easily recovered due to the low tear strength. The slight longer curing time (approx. 3 minutes) makes it more suitable for stationary preparations and in vitro tissue studies.
Kwik-Sil™ New Tip Design

Kwik-Sil™ and Kwik-Cast™ now come with a new tip design. The new tip is engineered to minimize leakage.
Kwik-CAST™ Applications
Kwik-CAST™ silicone is based on technology with vinyl terminated siloxane and platinum complex catalysts. In order to cure at room temperature, special cross linkers and high catalyst concentration are used. Although the high concentration of catalysts makes these products more costly than traditional RTV silicones and less attractive for general usage, they provide an excellent value in applications for the biological research field.
Benefits
- Low toxicity surgical adhesive
- Rapid cure time
- 6 Month shelf life
Applications
- Neuroscience and nerve studies
- Biomedical applications
Kwik-Sil and Kwik-Cast silicones have very low toxicity before, during and after curing. The by-product of curing is a small amount of hydrogen gas, which is much less toxic to cells than acetic acid or alcohol from traditional RTV silicone sealant systems.
Kwik-Sil and Kwik-Cast curing speed is hundreds of times faster than traditional RTV silicone adhesives. A curing time of a few minutes at room temperature is especially useful for encapsulation of live tissue or implanting into a live animal. Unlike many vinyl-based silicone adhesivess in which the platinum complex catalysts are easily poisoned by contamination from amines and animal tissue, Kwik-Sil and Kwik-Cast are not sensitive to contamination from animal tissue.
Kwik-Sil™ is a translucent, medium-viscosity silicone adhesive, developed for chronic peripheral nerve studies such as anterograde tracing with fluorescent indicators or electrode recording. Good adhesion and mechanical properties (tear strength and elongation) allow days of study without breaking of the bonding. Curing speed is very reproducible.
Kwik-Cast™ is a very low viscosity silicone sealant developed to embed peripheral nerves with electrodes for acute multi-fiber recordings. It flows easily, filling the small spaces around the nerve and leaving no channels through which peritoneal fluid can travel and thus short the nerve/electrode contact. Equally important is the ability of the material to flow into itself and create one continuous mass from underneath the nerve all the way to the top of the nerve/electrode contact to ensure long-term recording stability. Kwik-Cast is color-coded to make the mixing foolproof. The catalyst is yellow and the base is blue. When uniformly mixed, it is green. Kwik-Cast silicone sealant can be applied and cured underneath mineral oil. After recording, electrodes are easily recovered due to the low tear strength. The slight longer curing time (approx. 3 minutes) makes it more suitable for stationary preparations and in vitro tissue studies.
Curing Time
The properties of this biocompatible adhesive make it exceptionally useful for neuroscience applications, peripheral nerve studies and similar biomedical applications. These silicone elastomers also eliminate the mess and time involved in pre-mixing other commonly used formulations (such as Wacker SilGel® and Sylgard®). Each silicone elastomer is packaged in a double barrel syringe and is automatically mixed when pressed out of the mixer tip provided. The silicone sealant can then be applied directly to the tissue without further mixing. The curing time of these silicone adhesives is short, reliable and reproducible. This eliminating potential costly guesswork when using other tissue adhesives. The curing process does not produce any heat, which can cause tissue damage. WPI's silicone elastomers are much less toxic than dental silicone, because they contain no surfactant additives.
The silicone adhesive inside the syringes cannot be sterilized by any means including UV, gamma, H2O2, autoclaving or EtO. The exterior and the tips can be immersed in or wiped with 70/30 alcohol or you can use a liquid sterilant like Cidex OPA (WPI P/N 7364-4) or Rapicide OPA (WPI P/N 504611).
NOTE: Due to supply constraint, this product is currently non-returnable/non-refundable. For details, please refer to the WPI Terms & Conditions.
| SKU | KWIK-CAST |
|---|---|
| Options | Sterile |
Upsell Products
Kwik-Sil/Kwik-Cast Instruction Manual
Video
5 Reasons KWIK-SIL & KWIK-Cast Adhesive are Ideal for Neuroscience Application
KWIK-SIL versus KWIK-Cast, What's the Difference
FAQ
- I want to use KwikSil surgical glue in my "imaging" experiments in rats. How do I get rid of the bubbles completely, or at least reduce them as much as possible?
The bubbles are hydrogen gas that is released as a by-product of the curing process. The cure happens in such a short period that the gas can't escape. A slower cure time might help. To slow the cure process, cool the material before mixing.
- Can the adhesive be removed safely from rodent fur after use after it has cured?
If Kwik-Cast/Sil is applied to rodent fur, the cured adhesive can be removed from fur. Just gently and slowly pull the cured adhesive away from fur. Naturally, there are oils on fur and hair.
KWIK-CAST & KWIK-SIL Specifications
KWIK-CAST Silicone Sealant (two 5-mL syringes)
KWIK-SIL Silicone Adhesive Compound (two 5-mL syringes)
| Kwik-Sil | Kwik-Cast | |
| MIX RATIO | 1 to 1 | 1 to 1 |
| WORKING TIME | < 5 minutes* | 4 minutes |
| SETTING TIME (ROOM TEMP., 1:1 RATIO) | 5–10 minutes** | <10 minutes |
| CURE TIME | ~15 minutes | ~15 minutes |
| VISCOSITY, CPS | 15,000 | 10,000 |
| GUARANTEED SHELF LIFE AT 23 °C | 6 months | 6 months |
| VOLUME | 5 mL | 5 mL |
| NUMBER OF MIXING TIP | 10 | 10 |
| DEAD VOLUME OF THE MIXING TIP | <0.12 mL | <0.12 mL |
| NOTE | This is the consistency of pourable silicone rubber. | This is the consistency of chocolate syrup. |
| AFTER CURING 24 HOURS: | ||
| TEAR STRENGTH, PPI | 90 | 44 |
| ELONGATION % | 650 | 60 |
| DUROMETER (SHORE A-2) | 30 | 36 |
| COLOR | translucent | green |
| VOLUME RESISTIVITY, W/CM | 1x1015 | 1x1015 |
* 3 minutes average with about 90 seconds of liquidity
** no longer mixable at this point
Maura Casciola, Shu Xiao & Andrei G. Pakhomov. Damage-free peripheral nerve stimulation by 12-ns pulsed electric field. Scientific Reports 7, Article number: 10453.
https://www.nature.com/articles/s41598-017-10282-5 .
Tiran, E., Ferrier, J., Deffieux, T., Gennisson, J.-L., Pezet, S., Lenkei, Z., & Tanter, M. (2017). Transcranial Functional Ultrasound Imaging in Freely Moving Awake Mice and Anesthetized Young Rats without Contrast Agent. Ultrasound in Medicine & Biology, 43(8), 1679–1689. http://doi.org/10.1016/j.ultrasmedbio.2017.03.011
Lee, D., & Lee, A. K. (2017). Efficient Method for Whole-Cell Recording in Freely Moving Rodents Using Ultraviolet-Cured Collar-Based Pipette Stabilization. Cold Spring Harbor Protocols, 2017(4), pdb.prot095810. http://doi.org/10.1101/pdb.prot095810
Welle, T., Hoekstra, A. T., Daemen, I. A. J. J. M., Berkers, C. R., & Costa, M. O. (2017). Metabolic response of porcine colon explants to in vitro infection by Brachyspira hyodysenteriae: a leap into disease pathophysiology. Metabolomics, 13(7), 83. http://doi.org/10.1007/s11306-017-1219-6
Lee, D., & Lee, A. K. (2017). In Vivo Patch-Clamp Recording in Awake Head-Fixed Rodents. Cold Spring Harbor Protocols, 2017(4), pdb.prot095802. http://doi.org/10.1101/pdb.prot095802
Suwabe, T., & Bradley, R. M. (n.d.). Effects of 5-Hydroxytryptamine and Substance P on Neurons of the Inferior Salivatory Nucleus. http://doi.org/10.1152/jn.00859.2006
Suwabe, T., Fukami, H., & Bradley, R. M. (n.d.). Synaptic Responses of Neurons Controlling the Parotid and von Ebner Salivary Glands in Rats to Stimulation of the Solitary Nucleus and Tract. http://doi.org/10.1152/jn.01115.2007
Takakura, A. C., Colombari, E., Menani, J. V, & Moreira, T. S. (n.d.). Ventrolateral medulla mechanisms involved in cardiorespiratory responses to central chemoreceptor activation in rats.
Roberts, D. G., Johnsonbaugh, H. B., Spence, R. D., & MacKenzie-Graham, A. (2016). Optical Clearing of the Mouse Central Nervous System Using Passive CLARITY. Journal of Visualized Experiments, (112), e54025–e54025. http://doi.org/10.3791/54025
Lacin, E., Muller, A., Fernando, M., Kleinfeld, D., & Slesinger, P. A. (2016). Construction of Cell-based Neurotransmitter Fluorescent Engineered Reporters (CNiFERs) for Optical Detection of Neurotransmitters <em>In Vivo</em> Journal of Visualized Experiments, (111), e53290–e53290. http://doi.org/10.3791/53290
Su, X., Nickles, A., & Nelson, D. E. (2015). Preclinical assessment of potential interactions between botulinum toxin and neuromodulation for bladder micturition reflex. BMC Urology, 15(1), 50. http://doi.org/10.1186/s12894-015-0048-z
Burgalossi, A., von Heimendahl, M., & Brecht, M. (2014). Deep layer neurons in the rat medial entorhinal cortex fire sparsely irrespective of spatial novelty. Frontiers in Neural Circuits, 8, 74. http://doi.org/10.3389/fncir.2014.00074
Okubo, T. S., Mackevicius, E. L., & Fee, M. S. (2014). In vivo recording of single-unit activity during singing in zebra finches. Cold Spring Harbor Protocols, 2014(12), 1273–83. http://doi.org/10.1101/pdb.prot084624
Barnes, M. J., & McDougal, D. H. (2014). Leptin into the rostral ventral lateral medulla (RVLM) augments renal sympathetic nerve activity and blood pressure. Frontiers in Neuroscience, 8, 232. http://doi.org/10.3389/fnins.2014.00232
Roome, C. J., & Kuhn, B. (2014). Chronic cranial window with access port for repeated cellular manipulations, drug application, and electrophysiology. Frontiers in Cellular Neuroscience, 8, 379. http://doi.org/10.3389/fncel.2014.00379
Su, X., Nickles, A., & Nelson, D. E. (2013). Neuromodulation attenuates bladder hyperactivity in a rat cystitis model. BMC Urology, 13, 70. http://doi.org/10.1186/1471-2490-13-70
Pisauro, M. A., Dhruv, N. T., Carandini, M., & Benucci, A. (2013). Fast Hemodynamic Responses in the Visual Cortex of the Awake Mouse. Journal of Neuroscience, 33(46), 18343–18351. http://doi.org/10.1523/JNEUROSCI.2130-13.2013
Hromádka, T., Zador, A. M., & Deweese, M. R. (2013). Up states are rare in awake auditory cortex. Journal of Neurophysiology, 109(8), 1989–95. http://doi.org/10.1152/jn.00600.2012
Otchy, T. M., & Ӧlveczky, B. P. (2012). Design and Assembly of an Ultra-light Motorized Microdrive for Chronic Neural Recordings in Small Animals. Journal of Visualized Experiments, (69), e4314–e4314. http://doi.org/10.3791/4314
Salt, A. N., King, E. B., Hartsock, J. J., Gill, R. M., & O’Leary, S. J. (2012). Marker entry into vestibular perilymph via the stapes following applications to the round window niche of guinea pigs. Hearing Research, 283(1–2), 14–23. http://doi.org/10.1016/j.heares.2011.11.012
Levy, M., Schramm, A. E., & Kara, P. (2012). Strategies for mapping synaptic inputs on dendrites in vivo by combining two-photon microscopy, sharp intracellular recording, and pharmacology. Frontiers in Neural Circuits, 6, 101. http://doi.org/10.3389/fncir.2012.00101
Salt, A. N., Hartsock, J. J., Gill, R. M., Piu, F., & Plontke, S. K. (2012). Perilymph Pharmacokinetics of Markers and Dexamethasone Applied and Sampled at the Lateral Semi-Circular Canal. Journal of the Association for Research in Otolaryngology, 13(6), 771–783. http://doi.org/10.1007/s10162-012-0347-y
Niedzwiecki, D. J., & Movileanu, L. (2011). Monitoring Protein Adsorption with Solid-state Nanopores. Journal of Visualized Experiments, (58), e3560–e3560. http://doi.org/10.3791/3560
Zhang, Z., Liu, X., Morgan, D. A., Kuburas, A., Thedens, D. R., Russo, A. F., & Rahmouni, K. (2011). Neuronal receptor activity-modifying protein 1 promotes energy expenditure in mice. Diabetes, 60(4), 1063–71. http://doi.org/10.2337/db10-0692
Zhang, Z., Liu, X., Morgan, D. A., Kuburas, A., Thedens, D. R., Russo, A. F., & Rahmouni, K. (2011). Neuronal Receptor Activity–Modifying Protein 1 Promotes Energy Expenditure in Mice. Diabetes, 60(4).
Xie, C., & Wang, D. H. (2011). Inhibition of renin release by arachidonic acid metabolites, 12(s)-HPETE and 12-HETE: role of TRPV1 channels. Endocrinology, 152(10), 3811–9. http://doi.org/10.1210/en.2011-0141
Zhang, Q., Nishimura, D., Seo, S., Vogel, T., Morgan, D. A., Searby, C., … Wynshaw-Boris, A. (2011). No Title. Proceedings of the National Academy of Sciences of the United States of America, 108(51), 20678–83. http://doi.org/10.1073/pnas.1113220108
Wei, C.-C. C., Hase, N., Inoue, Y., Bradley, E. W., Yahiro, E., Li, M., … Kunori, Y. (2010). Mast cell chymase limits the cardiac efficacy of Ang I-converting enzyme inhibitor therapy in rodents. The Journal of Clinical Investigation, 120(4), 1229–39. http://doi.org/10.1172/JCI39345
Xie, C., & Wang, D. H. (2009). Ablation of transient receptor potential vanilloid 1 abolishes endothelin-induced increases in afferent renal nerve activity: mechanisms and functional significance. Hypertension, 54(6), 1298–305. http://doi.org/10.1161/HYPERTENSIONAHA.109.132167
Di Fiori, N., & Meller, A. (2009). Automated System for Single Molecule Fluorescence Measurements of Surface-immobilized Biomolecules. Journal of Visualized Experiments, (33), e1542. http://doi.org/10.3791/1542
Hromádka, T., Deweese, M. R., & Zador, A. M. (2008). Sparse representation of sounds in the unanesthetized auditory cortex. PLoS Biology, 6(1), e16. http://doi.org/10.1371/journal.pbio.0060016
Xie, C., Sachs, J. R., & Wang, D. H. (2008). Interdependent Regulation of Afferent Renal Nerve Activity and Renal Function: Role of Transient Receptor Potential Vanilloid Type 1, Neurokinin 1, and Calcitonin Gene-Related Peptide Receptors. Journal of Pharmacology and Experimental Therapeutics, 325(3), 751–757. http://doi.org/10.1124/jpet.108.136374
Dombeck, D. A., Khabbaz, A. N., Collman, F., Adelman, T. L., & Tank, D. W. (2007). Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron, 56(1), 43–57. http://doi.org/10.1016/j.neuron.2007.08.003
Rennaker, R. L., Miller, J., Tang, H., & Wilson, D. A. (2007). Minocycline increases quality and longevity of chronic neural recordings. Journal of Neural Engineering, 4(2), L1–L5. http://doi.org/10.1088/1741-2560/4/2/L01
Talman, W. T., Corr, J., Nitschke Dragon, D., & Wang, D. (2007). Parasympathetic stimulation elicits cerebral vasodilatation in rat. Autonomic Neuroscience, 133(2), 153–157. http://doi.org/10.1016/j.autneu.2006.12.002
Ruggeri, P., Brunori, A., Cogo, C. E., Storace, D., Di Nardo, F., & Burattini, R. (2006). Enhanced sympathetic reactivity associates with insulin resistance in the young Zucker rat. Am J Physiol Regul Integr Comp Physiol, 291, 376–382. http://doi.org/10.1152/ajpregu.00644.2005
Sirjani, D. B., Salt, A. N., Gill, R. M., & Hale, S. A. (2004). The influence of transducer operating point on distortion generation in the cochlea. The Journal of the Acoustical Society of America, 115(3), 1219. http://doi.org/10.1121/1.1647479
Duch, C., & Mentel, T. (2004). Activity affects dendritic shape and synapse elimination during steroid controlled dendritic retraction in Manduca sexta. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 24(44), 9826–37. http://doi.org/10.1523/JNEUROSCI.3189-04.2004
VanTeeffelen Jurgen, W. G. E., & Segal, S. S. (2000). Effect of motor unit recruitment on functional vasodilatation in hamster retractor muscle. The Journal of Physiology, 524(1), 267–278. http://doi.org/10.1111/j.1469-7793.2000.t01-1-00267.x