This website uses cookies to ensure you get the best experience on our website.
Read more
AMPLIFIER, 2CHANNEL DUAL INTRACELL ELECTROMETER
$7,500.00
Prices valid in USA, Canada, and PR only.
Order code
SYS-773

Prices valid in USA, Canada, and PR only.
2-Channel intracellular amplifier for dual and differential studies
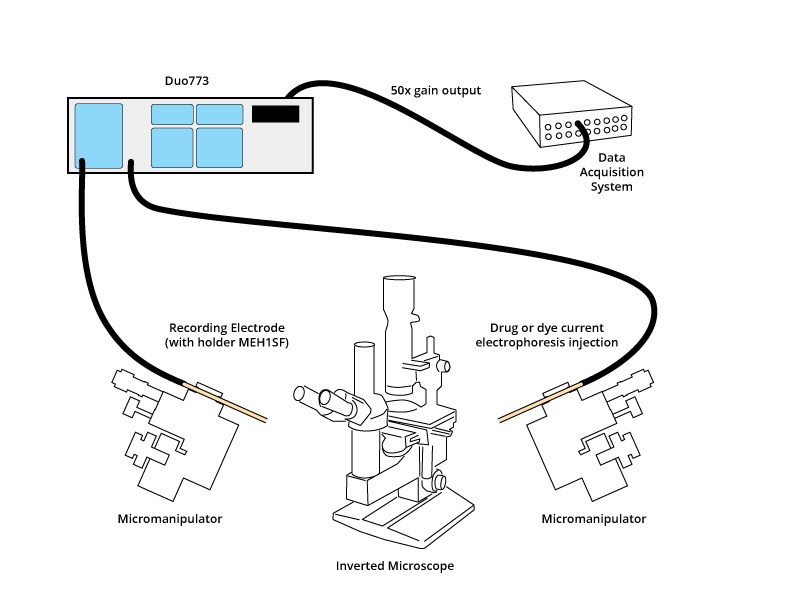
The Duo773 has two channes for differential or intracellular ISE with an integrated DC current generater and external control input. It has an integrated low pass filter. The bridge balance circuit nullifies the electrode voltage drop. And, it has an integrated "tickler" circuit with patent-pending electrode flutter technology. It has integrated test ports for each channel and dual capacitance compensations with output offse controls. The Duo773 comes complete with two probe headstages, normally one high impedance ona one low impedance.
Prices valid in USA, Canada, and PR only.
See what you need to know before you buy an amplifier.
Benefits
- Dual channel, single ended recording
- Differential recording
- Bridge circuit nulls electrode voltage drop
- Assign low pass filter to either channel
- Very high impedance channel can be used with intracellular ISE
Applications
- Intracellular electrophysiology using sharp micropipettes
- Brain slice intracellular recording
- In vivo intracellular recording from brain and spinal cord
For intracellular dual or differential studies, the Duo773 has separate negative capacity controls and built-in active filtering that allows the precise balancing of time constants for artifact-free differential measurement. Comes complete with two probe headstages, 1015Ω and 1011Ω probes to monitor signals from ion-specific micro-electrodes as well as KCl-filled electrodes.
Headstage for precise positioning
Two gold-plated, epoxy sealed miniature active probes can be positioned directly to the measurement site. Microelectrode holders containing an Ag/AgCl electrochemical half-cells plug directly into the probes. Stray capacitance can be reduced by placing the included driven guard shield over the microelectrode holder at the end of the probe.
Capacity compensation
Channel A can compensate up to 10 pF of electrode shunt capacity and Channel B can compensate up to 50 pF.
Tickler circuit for penetration
A Tickler Circuit assists in cell penetration. The frequency and amplitude of the oscillations may be varied for differences in membrane thickness or cell size. The duration of tickle can be controlled either by using the momentary switch, a foot switch, or by applying a signal to the remote tickler input.
Active filters
Low pass settings on a -40 dB/decade active filter vary the cutoff from 1 to 30 kHz. Either probe or bridge outputs may be selected for filtering.
Current injection
Channel B can eject current through the microelectrode by applying a command signal to the stimulus input connector. The resulting output from the probe will be a constant current replica of the input signal. Two ranges of current delivery are provided: 50 nA and 500 nA or by an external source. This source can be useful for delivering hyperpolarizing currents to stabilize the cell membrane potential and as a holding current for microiontophoresis.
Bridge balance
Subtracts the excess electrode voltage associated with delivering current through the recording micropipette. Electrode resistances up to 1000 MΩ can be balanced in two ranges. The balanced signal is available from x10 or x50 front panel output connectors.
Independent outputs
The Duo773 has an output for each probe independent of gain filtering or balancing. In addition the Duo773 has a 10x and a 50x output for easy integration to most data acquisition programs.
Typical setup
See Dri-Ref reference electrodes.
Optional holders for intracellular amplifiers
Other microelectrode holders
| SKU | SYS-773 |
|---|
| HEADSTAGE (PROBE) | 712P (red, port B) | 715P (blue, port A) |
| ACTIVE PROBE INPUT IMPEDANCE | >1011 Ω | 1015 Ω |
| GAIN | x1, x10 | x1 |
| OUTPUT RESISTANCE | 100 Ω | 100 Ω |
| OUTPUT VOLTAGE RANGE | ±10 V | ±10 V |
| MAXIMUM INPUT VOLTAGE | ±15 V | ±15 V |
| PROBE LEAKAGE CURRENT | 5 X 10-12 A | 10-14 A |
| DC POSITION ADJUST RANGE | ± 300 mV | ± 300 mV |
| ELECTRODE RESISTANCE TEST CURRENT | 1 nA | 1 pA, 1 nA selectable |
| INPUT CAPACITY COMPENSATION | +10 to -50 pF | 0 to -10 pF |
| NOISE Input shorted 712P 20 MΩ carbon resistor |
<50 µV p-p 10 kHz bandwidth <200 µV p-p 10 kHz bandwidth |
<50 µV p-p 10 kHz bandwidth <200 µV p-p 10 kHz bandwidth |
| RISE TIME 10-90% direct input small signal 10-90% through 20 MΩ (-C "on") |
1 µs, typical 25 µs, typical |
|
| CURRENT INJECTION (712P only)** Internal DC Current Externally commanded Current 712P (red, port B) External current command factor Current monitor Compliance Bridge balance Bridge amplifier gain |
± 50 nA low range, ± 500 nA high range ± 500 nA low range, ±5 µA high range 20 mV/nA low range, 2 mV/nA high range 100mV/nA low range, 10mV/nA high range 3V low range, 10V high range 0-100 MΩ, 0-1000 MΩ x 10, x 50 |
n/a |
| LOW PASS FILTER | 40 dB/decade, continuously variable 1-30 kHz | |
| Fuse (Older models) | 120 V: 0.5 A, fast, 0.25x1.25” USA 230 V: 0.25 A, fast, 0.25x1.25” USA |
|
| Fuse (2019 models) | 120 V: 0.5 A, fast, 5 x 20 mm metric 230 V: 0.25 A, fast, 5 x 20 mm metric |
|
| METER SECTION Display Ranges Accuracy and resolution |
3.5-digit LED 200 mV, 2000 mV, 20 V, 200 nA, 2000 nA 1 digit |
|
| DIMENSIONS: Instrument Probe |
17 x 5.25 x 10 in. (43 x 13 x 25 cm) Diameter: 12 mm Length: 34mm |
|
| POWER | 95-135 V or 220-240 V, 50/60 Hz | |
| SHIPPING WEIGHT | 15 lb. (7 kg) | |
| CERTIFICATION | CE, CSA | |
* Although injected currents are “constant,” the maximum current in a given situation will always be limited by the system compliance of 10 V.
**The 712P headstage may be used on either A or B channels, however Current Injection specifications do not apply when used on channel A. The 715P headstage may not be used on the B channel.
PLants Employed As SEnsor Devices | Projects | FP7-ICT | CORDIS | European Commission. (n.d.). Retrieved November 27, 2018, from https://cordis.europa.eu/project/rcn/103686_en.html
Zhang, J., Chen, M., Li, B., Lv, B., Jin, K., Zheng, S., … Long, C. (2016). Altered striatal rhythmic activity in cylindromatosis knock-out mice due to enhanced GABAergic inhibition. Neuropharmacology, 110, 260–267. https://doi.org/10.1016/j.neuropharm.2016.06.021
Cros, C., Chaigne, S., Pascarel-Auclerc, C., Benoist, D., Walton, R., Pasdois, P., … Brette, F. (2016). 0514 : Isolation of cardiac myocytes from human heart. Archives of Cardiovascular Diseases Supplements, 8(3), 230. https://doi.org/10.1016/S1878-6480(16)30430-X
Mañé, N., Viais, R., Martínez-Cutillas, M., Gallego, D., Correia-de-Sá, P., & Jiménez, M. (2016). Inverse gradient of nitrergic and purinergic inhibitory cotransmission in the mouse colon. Acta Physiologica, 216(1), 120–131. https://doi.org/10.1111/apha.12599
Chang, J.-H., Cheng, P.-Y., Hsu, C.-H., Chen, Y.-C., & Hong, P.-D. (2016). Effects of Acetaminophen on Left Atrial Contractility. Acta Cardiologica Sinica, 32(4), 485–490. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/27471362
Huo, Q., Chen, M., He, Q., Zhang, J., Li, B., Jin, K., … Yang, L. (2016). Prefrontal Cortical GABAergic Dysfunction Contributes to Aberrant UP-State Duration in APP Knockout Mice. Cerebral Cortex (New York, N.Y. : 1991). https://doi.org/10.1093/cercor/bhw218
Spong, K. E., Rodríguez, E. C., & Robertson, R. M. (2016). Spreading depolarization in the brain of Drosophila is induced by inhibition of the Na+/K+-ATPase and mitigated by a decrease in activity of protein kinase G. Journal of Neurophysiology, 116(3).
Mañé, N., Jiménez-Sábado, V., & Jiménez, M. (2016). BPTU, an allosteric antagonist of P2Y1 receptor, blocks nerve mediated inhibitory neuromuscular responses in the gastrointestinal tract of rodents. Neuropharmacology, 110, 376–385. https://doi.org/10.1016/j.neuropharm.2016.07.033
Bredeloux, P., Finday, I., Pasqualin, C., Yu, A., & Maupoil, V. (2016). 0194 : Functional consequences of -adrenergic receptors activation in the rat pulmonary veins and left atria. Archives of Cardiovascular Diseases Supplements, 8(3). https://doi.org/10.1016/S1878-6480(16)30431-1
Coskun, D., Britto, D. T., Kochian, L. V., & Kronzucker, H. J. (2016). How high do ion fluxes go? A re-evaluation of the two-mechanism model of K+ transport in plant roots. Plant Science, 243, 96–104. https://doi.org/10.1016/j.plantsci.2015.12.003
Magown, P., Shettar, B., Zhang, Y., & Rafuse, V. F. (2015). Direct optical activation of skeletal muscle fibres efficiently controls muscle contraction and attenuates denervation atrophy. Nature Communications, 6(1), 8506. https://doi.org/10.1038/ncomms9506
Yi, F., Ling, T.-Y., Lu, T., Wang, X.-L., Li, J., Claycomb, W. C., … Lee, H.-C. (2015). Down-regulation of the small conductance calcium-activated potassium channels in diabetic mouse atria. The Journal of Biological Chemistry, 290(11), 7016–7026. https://doi.org/10.1074/jbc.M114.607952
van der Schoot, C., & Rinne, P. L. H. (2015). Mapping Symplasmic Fields at the Shoot Apical Meristem Using Iontophoresis and Membrane Potential Measurements (pp. 157–171). https://doi.org/10.1007/978-1-4939-1523-1_11
Chatterjee, S. K., Das, S., Maharatna, K., Masi, E., Santopolo, L., Mancuso, S., & Vitaletti, A. (2015). Exploring strategies for classification of external stimuli using statistical features of the plant electrical response. Journal of The Royal Society Interface, 12(104), 20141225–20141225. https://doi.org/10.1098/rsif.2014.1225
Pan, X., Zhang, Z., Huang, Y.-Y., Zhao, J., & Wang, L. (2015). Electrophysiological Effects of Dexmedetomidine on Sinoatrial Nodes of Rabbits. Acta Cardiologica Sinica, 31(6), 543–549. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/27122920
Huang, J., Dosdall, D. J., Cheng, K., Li, L., Rogers, J. M., & Ideker, R. E. (2014). The Importance of Purkinje Activation in Long Duration Ventricular Fibrillation. Journal of the American Heart Association, 3(1), e000495. https://doi.org/10.1161/JAHA.113.000495
Altamirano, F., Eltit, J. M., Robin, G., Linares, N., Ding, X., Pessah, I. N., … López, J. R. (2014). Ca 2+ Influx via the Na + /Ca 2+ Exchanger Is Enhanced in Malignant Hyperthermia Skeletal Muscle. Journal of Biological Chemistry, 289(27), 19180–19190. https://doi.org/10.1074/jbc.M114.550764
Altamirano, F., Perez, C. F., Liu, M., Widrick, J., Barton, E. R., Allen, P. D., … Lopez, J. R. (2014). Whole Body Periodic Acceleration Is an Effective Therapy to Ameliorate Muscular Dystrophy in mdx Mice. PLoS ONE, 9(9), e106590. https://doi.org/10.1371/journal.pone.0106590
Liu, D.-H., Huang, X., Guo, X., Meng, X.-M., Wu, Y.-S., Lu, H.-L., … Xu, W.-X. (2014). Voltage Dependent Potassium Channel Remodeling in Murine Intestinal Smooth Muscle Hypertrophy Induced by Partial Obstruction. PLoS ONE, 9(2), e86109. https://doi.org/10.1371/journal.pone.0086109
Mousavi, S. A. R., Chauvin, A., Pascaud, F., Kellenberger, S., & Farmer, E. E. (2013). GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature, 500(7463), 422–426. https://doi.org/10.1038/nature12478
Altamirano, F., Valladares, D., Henríquez-Olguín, C., Casas, M., López, J. R., Allen, P. D., & Jaimovich, E. (2013). Nifedipine Treatment Reduces Resting Calcium Concentration, Oxidative and Apoptotic Gene Expression, and Improves Muscle Function in Dystrophic mdx Mice. PLoS ONE, 8(12), e81222. https://doi.org/10.1371/journal.pone.0081222
Chen, J., Du, L., Xiao, Y.-T., & Cai, W. (2013). Disruption of interstitial cells of Cajal networks after massive small bowel resection. World Journal of Gastroenterology, 19(22), 3415. https://doi.org/10.3748/wjg.v19.i22.3415
Eltit, J. M., Ding, X., Pessah, I. N., Allen, P. D., & Lopez, J. R. (2013). Nonspecific sarcolemmal cation channels are critical for the pathogenesis of malignant hyperthermia. The FASEB Journal, 27(3), 991–1000. https://doi.org/10.1096/fj.12-218354
Hafke, J. B., Höll, S.-R., Kühn, C., & van Bel, A. J. E. (2013). Electrophysiological approach to determine kinetic parameters of sucrose uptake by single sieve elements or phloem parenchyma cells in intact Vicia faba plants. Frontiers in Plant Science, 4, 274. https://doi.org/10.3389/fpls.2013.00274
Guo, X., Huang, X., Wu, Y., Liu, D., Lu, H., Kim, Y., … Xu, W. (2012). Down-Regulation of Hydrogen Sulfide Biosynthesis Accompanies Murine Interstitial Cells of Cajal Dysfunction in Partial Ileal Obstruction. PLoS ONE, 7(11), e48249. https://doi.org/10.1371/journal.pone.0048249
UEHLEIN, N., SPERLING, H., HECKWOLF, M., & KALDENHOFF, R. (2012). The Arabidopsis aquaporin PIP1;2 rules cellular CO2 uptake. Plant, Cell & Environment, 35(6), 1077–1083. https://doi.org/10.1111/j.1365-3040.2011.02473.x
Altamirano, F., López, J. R., Henríquez, C., Molinski, T., Allen, P. D., & Jaimovich, E. (2012). Increased Resting Intracellular Calcium Modulates NF-κB-dependent Inducible Nitric-oxide Synthase Gene Expression in Dystrophic mdx Skeletal Myotubes. Journal of Biological Chemistry, 287(25), 20876–20887. https://doi.org/10.1074/jbc.M112.344929
Hamaguchi, K., Yamamoto, N., Nakagawa, T., Furuyashiki, T., Narumiya, S., & Ito, J. (2012). Role of PGE-type receptor 4 in auditory function and noise-induced hearing loss in mice. Neuropharmacology, 62(4), 1841–1847. https://doi.org/10.1016/j.neuropharm.2011.12.007
Armstrong, G. A. B., Rodríguez, E. C., & Meldrum Robertson, R. (2012). Cold hardening modulates K+ homeostasis in the brain of Drosophila melanogaster during chill coma. Journal of Insect Physiology, 58(11), 1511–1516. https://doi.org/10.1016/J.JINSPHYS.2012.09.006
Tsai, C.-F., Chen, Y.-C., Lin, Y.-K., Chen, S.-A., & Chen, Y.-J. (2011). Electromechanical effects of the direct renin inhibitor (aliskiren) on the pulmonary vein and atrium. Basic Research in Cardiology, 106(6), 979–993. https://doi.org/10.1007/s00395-011-0206-8
Han, Y., Huang, X., Guo, X., Wu, Y., Liu, D., Lu, H., … Xu, W. (2011). Evidence that endogenous hydrogen sulfide exerts an excitatory effect on gastric motility in mice. European Journal of Pharmacology, 673(1–3), 85–95. https://doi.org/10.1016/j.ejphar.2011.10.018
Haugan, B. M., Halberg, K. A., Jespersen, Å., Prehn, L. R., & Møbjerg, N. (2010). Functional characterization of the vertebrate primary ureter: Structure and ion transport mechanisms of the pronephric duct in axolotl larvae (Amphibia). BMC Developmental Biology, 10(1), 56. https://doi.org/10.1186/1471-213X-10-56
Li, H., Ding, X., Lopez, J. R., Takeshima, H., Ma, J., Allen, P. D., & Eltit, J. M. (2010). Impaired Orai1-mediated Resting Ca 2+ Entry Reduces the Cytosolic [Ca 2+ ] and Sarcoplasmic Reticulum Ca 2+ Loading in Quiescent Junctophilin 1 Knock-out Myotubes. Journal of Biological Chemistry, 285(50), 39171–39179. https://doi.org/10.1074/jbc.M110.149690
Ruonala, R., Rinne, P. L. H., Kangasjarvi, J., & van der Schoot, C. (2008). CENL1 Expression in the Rib Meristem Affects Stem Elongation and the Transition to Dormancy in Populus. THE PLANT CELL ONLINE, 20(1), 59–74. https://doi.org/10.1105/tpc.107.056721
Keller, C. P., Barkosky, R. R., Seil, J. E., Mazurek, S. A., & Grundstad, M. L. (2008). The electrical response of Phaseolus vulgaris roots to abrupt exposure to hydroquinone. Plant Signaling & Behavior, 3(9), 633–640. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19513254
McDonnell, B., Hamilton, R., Fong, M., Ward, S. M., & Keef, K. D. (2008). Functional evidence for purinergic inhibitory neuromuscular transmission in the mouse internal anal sphincter. American Journal of Physiology-Gastrointestinal and Liver Physiology, 294(4), G1041–G1051. https://doi.org/10.1152/ajpgi.00356.2007
Lew, R. R. (2007). Ionic currents and ion fluxes in Neurospora crassa hyphae. Journal of Experimental Botany, 58(12), 3475–3481. https://doi.org/10.1093/jxb/erm204
Patterson, E., Po, S. S., Scherlag, B. J., & Lazzara, R. (2005). Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm, 2(6), 624–631. https://doi.org/10.1016/j.hrthm.2005.02.012
Cho, S. Y., Beckett, E. A., Baker, S. A., Han, I., Park, K. J., Monaghan, K., … Koh, S. D. (2005). A pH-sensitive potassium conductance (TASK) and its function in the murine gastrointestinal tract. The Journal of Physiology, 565(Pt 1), 243–259. https://doi.org/10.1113/jphysiol.2005.084574
Kreindler, J. L., Jackson, A. D., Kemp, P. A., Bridges, R. J., & Danahay, H. (2005). Inhibition of chloride secretion in human bronchial epithelial cells by cigarette smoke extract. American Journal of Physiology-Lung Cellular and Molecular Physiology, 288(5), L894–L902. https://doi.org/10.1152/ajplung.00376.2004
Verheule, S., Sato, T., Everett, T., Engle, S. K., Otten, D., Rubart-von der Lohe, M., … Olgin, J. E. (2004). Increased Vulnerability to Atrial Fibrillation in Transgenic Mice With Selective Atrial Fibrosis Caused by Overexpression of TGF-β1. Circulation Research, 94(11), 1458–1465. https://doi.org/10.1161/01.RES.0000129579.59664.9d
Hwang, H.-R., Shen, Y.-F., Chen, Y.-C., Liu, C.-P., & Lin, C.-I. (2004). Effects of cyclopiazonic acid on triggered activities in ventricular muscle and cardiomyocytes isolated from hamster hearts. The Chinese Journal of Physiology, 47(3), 137–142. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15612531
Ermilov, L. G., Schmalz, P. F., Miller, S. M., & Szurszewski, J. H. (2004). PACAP modulation of the colon-inferior mesenteric ganglion reflex in the guinea pig. The Journal of Physiology, 560(1), 231–247. https://doi.org/10.1113/jphysiol.2004.070060
Bruusgaard, J. C., Liestøl, K., Ekmark, M., Kollstad, K., & Gundersen, K. (2003). Number and spatial distribution of nuclei in the muscle fibres of normal mice studied in vivo. The Journal of Physiology, 551(Pt 2), 467–478. https://doi.org/10.1113/jphysiol.2003.045328
Lennon, V. A., Ermilov, L. G., Szurszewski, J. H., & Vernino, S. (2003). Immunization with neuronal nicotinic acetylcholine receptor induces neurological autoimmune disease. The Journal of Clinical Investigation, 111(6), 907–913. https://doi.org/10.1172/JCI17429
Chen, Y. J., Chen, S. A., Chang, M. S., & Lin, C. I. (2000). Arrhythmogenic activity of cardiac muscle in pulmonary veins of the dog: implication for the genesis of atrial fibrillation. Cardiovascular Research, 48(2), 265–273. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11054473
Ward, S. M., Beckett, E. A., Wang, X., Baker, F., Khoyi, M., & Sanders, K. M. (2000). Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 20(4), 1393–1403. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10662830
Kilb, W., & Schlue, W. R. (1999). Mechanism of the kainate-induced intracellular acidification in leech Retzius neurons. Brain Research, 824(2), 168–182. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10196447
Hara, M., Shvilkin, A., Rosen, M. R., Danilo, P., & Boyden, P. A. (1999). Steady-state and nonsteady-state action potentials in fibrillating canine atrium: abnormal rate adaptation and its possible mechanisms. Cardiovascular Research, 42(2), 455–469. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10533581
Muto, S., Asano, Y., Seldin, D., & Giebisch, G. (1999). Basolateral Na+ pump modulates apical Na+ and K+ conductances in rabbit cortical collecting ducts. The American Journal of Physiology, 276(1 Pt 2), F143-58. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9887090
Hara, M., Danilo, P. R., & Rosen, M. R. (1998). Effects of gonadal steroids on ventricular repolarization and on the response to E4031. Undefined. Retrieved from https://www.semanticscholar.org/paper/Effects-of-gonadal-steroids-on-ventricular-and-on-Hara-Danilo/1be02ac45630bf87ec224f8484890f68981572e3
Welsh, D. G., Jackson, W. F., & Segal, S. S. (1998). Oxygen induces electromechanical coupling in arteriolar smooth muscle cells: a role for L-type Ca 2+ channels. American Journal of Physiology-Heart and Circulatory Physiology, 274(6), H2018–H2024. https://doi.org/10.1152/ajpheart.1998.274.6.H2018
Kuwana, S., Okada, Y., & Natsui, T. (1998). Effects of extracellular calcium and magnesium on central respiratory control in the brainstem-spinal cord of neonatal rat. Brain Research, 786(1–2), 194–204. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9555011
Botha, C. E. J., & Cross, R. H. M. (1997). Plasmodesmatal frequency in relation to short-distance transport and phloem loading in leaves of barley (Hordeum vulgare). Phloem is not loaded directly from the symplast. Physiologia Plantarum, 99(3), 355–362. https://doi.org/10.1111/j.1399-3054.1997.tb00547.x
Keef, K. D., Murray, D. C., Sanders, K. M., & Smith, T. K. (1997). Basal release of nitric oxide induces an oscillatory motor pattern in canine colon. The Journal of Physiology, 499 ( Pt 3)(Pt 3), 773–786. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9130172
Felle, H. H., & Hepler, P. K. (1997). The Cytosolic Ca2+ Concentration Gradient of Sinapis alba Root Hairs as Revealed by Ca2+-Selective Microelectrode Tests and Fura-Dextran Ratio Imaging. Plant Physiology, 114(1), 39–45. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12223687
Lu, G., Qian, X., Berezin, I., Telford, G. L., Huizinga, J. D., & Sarna, S. K. (1997). Inflammation modulates in vitro colonic myoelectric and contractile activity and interstitial cells of Cajal. The American Journal of Physiology, 273(6 Pt 1), G1233-45. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9435548
Wang, R., & Crawford, N. M. (1996). Genetic identification of a gene involved in constitutive, high-affinity nitrate transport in higher plants. Proceedings of the National Academy of Sciences of the United States of America, 93(17), 9297–9301. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8799195
Woodruff, R. I., & Telfer, W. H. (1994). Steady-state gradient in calcium ion activity across the intercellular bridges connecting oocytes and nurse cells inHyalophora cecropia. Archives of Insect Biochemistry and Physiology, 25(1), 9–20. https://doi.org/10.1002/arch.940250103
Keef, K. D., Du, C., Ward, S. M., McGregor, B., & Sanders, K. M. (1993). Enteric inhibitory neural regulation of human colonic circular muscle: Role of nitric oxide. Gastroenterology, 105(4), 1009–1016. https://doi.org/10.5555/URI:PII:0016508593909437
Stark, M. E., Bauer, A. J., & Szurszewski, J. H. (1991). Effect of nitric oxide on circular muscle of the canine small intestine. The Journal of Physiology, 444, 743–761. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1688034
Butt, A. M., Jones, H. C., & Abbott, N. J. (1990). Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. The Journal of Physiology, 429, 47–62. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2277354
Wright, J. P., Fisher, D. B., Kelling, F., Furch, A. C. U., Gaupels, F., & Bel, A. J. E. van. (1981). Measurement of the Sieve Tube Membrane Potential. PLANT PHYSIOLOGY, 67(4), 845–848. https://doi.org/10.1104/pp.67.4.845