This website uses cookies to ensure you get the best experience on our website.
Read more
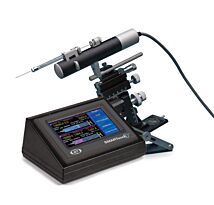
Microinjectors
As low as
$4,500.00
Only %1 left
Prices valid in USA, Canada, and PR only.
Order code
Price range: $4,500 - $9,800

Prices valid in USA, Canada, and PR only.
For All Your Microinjection Needs
WPI's new generation pressure microinjectors (the PV850, µPUMP, and MICRO-ePUMP) are quickly replacing the popular legacy line of pressure injectors (the PV820 and PV830). Our three new injectors facilitate any type of microinjecion that involves glass micropipettes, conveniently paired with a compensation pressure function to prevent capillary action.
Now featuring: Narishige & TransferMan-compatible capillary holder included with each unit!
To learn more about our warranty options, click here.
Prices valid in USA, Canada, and PR only.
New Generation of Pressure Injectors
- MICRO-ePUMP - Microinjector with Integrated Cell Penetrator and Internal Pressure Source
- μPUMP - Microinjector with Internal Pressure Source
- PV850 - Microinjector for Use with External Pressure Source*
*If you currently have either the µPUMP or PV850 and are interested in adding electroporation fuctionality to your system, check-out our MICRO-ePORE™.
Microinjectors For All Your Microinjection Applications
Designed to simplify intracellular injection and a variety of other microinjection tasks, the WPI introduces three new microinjectors:
| Type | MICRO-ePUMP |
uPUMP |
PV850 |
| Regulated compensation and injection pressure | |||
| Pressure output: 0.3–87 PSI | |||
| Low volume tubing assembly | |||
| Inject using foot switch (included) | |||
| Inject using a TTL pulse from external computer or pulse generator (Cable to connect to pulse generator included) |
|||
| Inject manually using touch screen interface | |||
| Integrated pressure source | |||
| 2-Stage foot switch to activate voltage for injection and MICRO-ePORETM |
|||
Integrated MICRO-ePORETM Technology
|
A common method of microinjection utilizes a glass micropipette tip to inject a liquid substance into an intra- or intercellular space. The glass microinjection needle tip can vary in size from sub-micron to about 5-6µm in diameter. Common applications include transgenic modification, where altered genetic material is introduced into an embryonic cell. Our microinjectors have been often used to modify various animal models such as mice, rats, fish (zebrafish, medaka, etc.), C. elegans, rabbits, and even larger models such as cattle, sheep and pigs.
Benefits 
- Intuitive user-interface, with touch screen and tailored knob adjustments
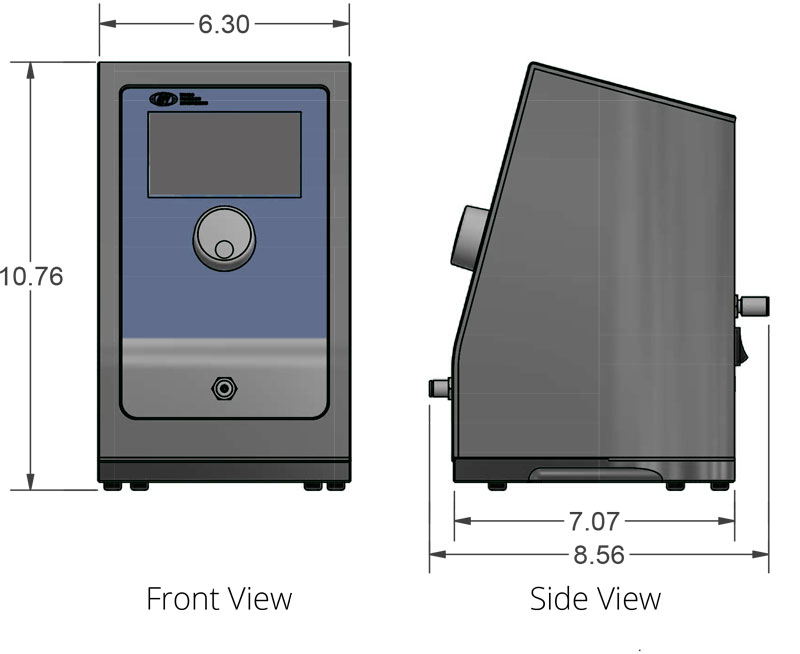
- Small footprint for optimized bench space
- High precision for reproducible injections – microliter to picoliter scale
- All inclusive system (tubing, footswitch, capillary holder with various gasket fittings)
- Plug-and-play system: little-to-no preparation required to begin working as quickly as possible
- Compensation pressure feature omits capillary action for complete control over your sample
- Option to use a range of micromanipulators with both with our standard 6.35mm pipette holder, and the slimmer 4mm holders, commonly paired with hydraulic manipulators.
- Premium Warranty Available
Applications
- Microinjection of diverse compounds and biomolecules
- Electron transfer kinetics on mono and multilayer graphene
- Creation of transgenic animal lines
- No length limitation (100’s of kb’s of DNA) on the transfer of foreign genes

- No length limitation (100’s of kb’s of DNA) on the transfer of foreign genes
 Injecting substances into cells
Injecting substances into cells
- Nucleic acids
- Organelles
- Kinases
- Histochemical markers (such as horseradish peroxidase or fluorescent yellow)
- Proteins, antibodies
- Metabolites
- Ions
- Microperfusion in a single cell or group of cells
- Analysis of early development processes like gastrulation, organogenesis, etc.
All Your Microinjection Accessories
Let WPI be your one-stop shop for all your microinjection needs. We offer any accessory you may need alongside your microinjector, from microscopes, manipulators, to pre-pulled glass pipettes.
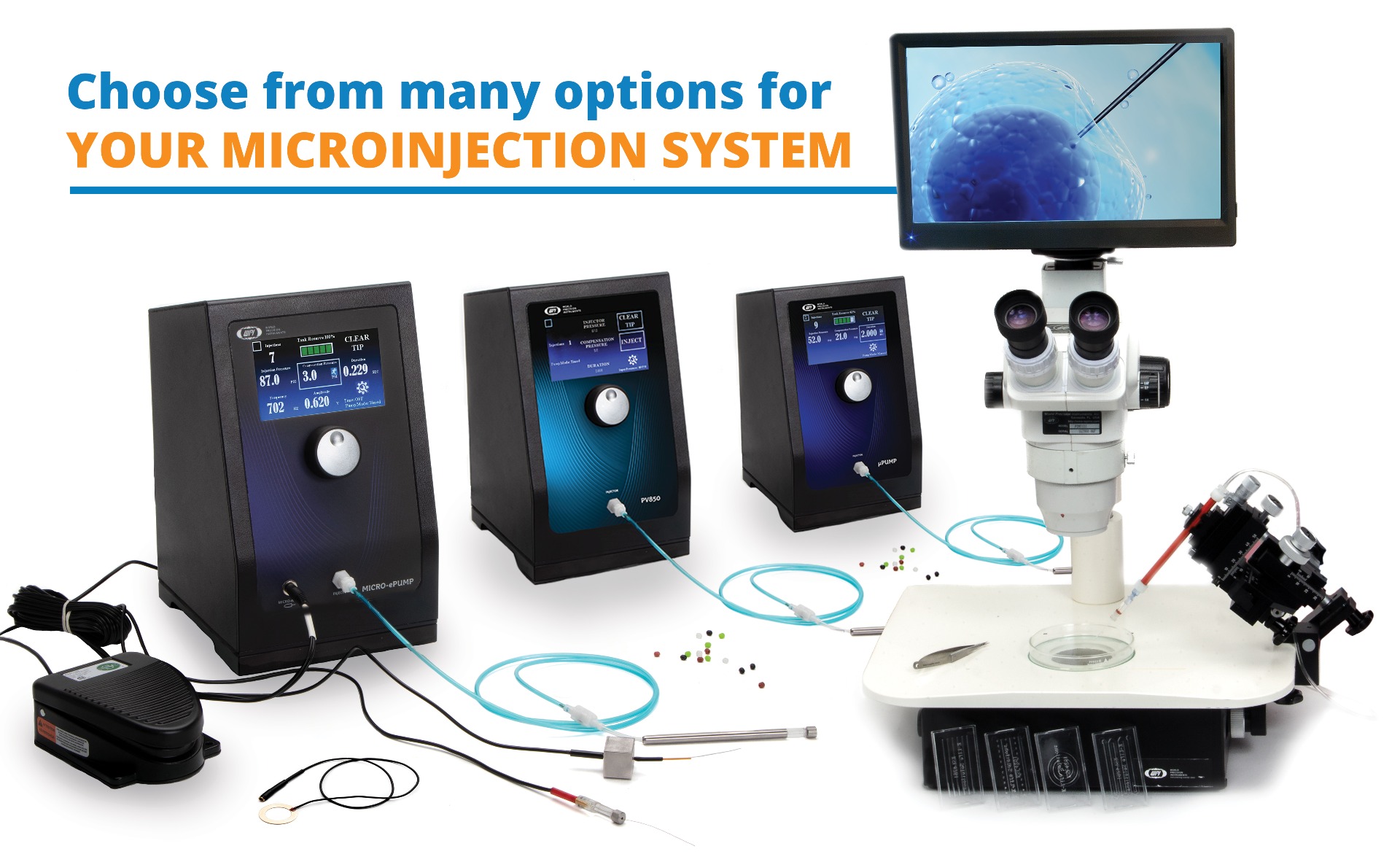
(1) MICRO-ePUMP
(1) Microinjector with Integrated Cell Penetrator and Internal Pressure Source.
(2) µPUMP
(2) Microinjector with Internal Pressure Source.
(3) PV850
(3) Microinjector with External Pressure Source (pressure source not included).
WPI Microinjectors use carefully regulated air pressure for injecting cells with fluid-based samples. Injected volumes range from picoliters to nanoliters. The front-facing port supplies positive pressure for rapid, and controlled injections, while automatically switching to a low- positive compensation pressure between injection pulses to prevent fluid uptake through capillary action.
Timing, injection pressure and compensation pressure are adjusted independently using the touch screen interface. Time intervals can range from 10milliseconds to 2 seconds, where the injection pressure interval is triggered by using a foot switch, computer-controlled TTL pulse, or by manual selection on the unit display (MICRO-ePUMP uses only a foot switch or manual trigger selection).
WPI’s MICRO-ePORETM Pinpoint Cell Penetrating technology is embedded inside the MICRO-ePUMP. When the researcher enables this functionality, it delivers a highly localized voltage signal to a targeted injection site on a cell surface to facilitate tip penetration with minimal trauma. The researcher determines the amplitude and frequency of the signal that best suits the application. The signal originates in the control box, and it is transmitted through the electrode interface cable to the microelectrode holder. A silver electrode wire is used to transmit the signal to the cell surface. A reference electrode is used to place the media at 0.0 V potential with reference to the generated voltage.
Our µPUMP provides an internal pressure source and recharging feature similar to the Micro-ePUMP, without electroporation capability. Some applications or cell types to not require the use of electroporation, so this option is perfect for those looking for a straightforward option that offers the convenience of an internal pressure source and regulation system.
The PV850 is our simplest, most economical option of WPI's new generation Microinjection systems. With its external pressure requirement, for those with a built-in pressure line on their tabletop, the set-up is as simple as the use of this system. If you are new to Microinjection, do not require electroporation, and have an external pressure source available, this option may be right for you!
System Components
| What is included with the system | Qty | MICRO-ePUMP | uPUMP | PV850 |
| Injector | 1 | Yes | Yes | Yes |
| 2-Step Foot Switch | 1 | Yes | Yes | Yes |
| 99164 MICRO-ePORETM Injection Assembly | 1 | Yes | ||
| 99788 MICRO-ePUMP Ground Cable | 1 | Yes | ||
| AC/DC 24 V Power Adapter | 1 | Yes | Yes | Yes |
| Capillary kit includes | 300744 | 300753 | 300753 | |
| 75122-110 1.0 mm Pipette Gaskets (green) | 4 | Yes | Yes | Yes |
| 75122-210 1.2 mm Pipette Gaskets (black) | 4 | Yes | Yes | Yes |
| 75122-310 1.5 mm Pipette Gaskets (red) | 4 | Yes | Yes | Yes |
| 75122-410 1.65 mm Pipette Gaskets (white) | 4 | Yes | Yes | Yes |
| 802828 O-ring Bunna N-002 | 2 | Yes | Yes | Yes |
| 75123 Pipette Handle Assembly | 1 | Yes | Yes | Yes |
| 98985 4MM Pipette Handle Assembly | 1 | Yes | Yes | Yes |
| 75125 Pipette Holder | 1 | Yes | Yes | Yes |
| 99862 Tubing Assembly | 1 | Yes | Yes | Yes |
| 99865 Adapter Tube Assembly | 1 | Yes | Yes | Yes |
| CBL102 6’ Cable, 3.5 mm Mini Phone Plug to BNC | 1 | No | Yes | Yes |
| 803130 Stereo Splitter Cable, 3.5 mm | 1 | No | Yes | Yes |
| Input kit for Picopumps includes | 1 | 3116 | ||
| 0.25” NPT Fitting for Nitrogen Tank Regulator | 1 | Yes | ||
| 10’ Hard Tubing | 1 | Yes | ||
| Vacuum Fitting | 1 | Yes |
| SKU | VAR-MICRO-ePUMP |
|---|
Documents
- MICRO-ePUMP Manual
- µPUMP Manual
- PV850 Manual
- Microinjectors Brochure
- MICRO-ePUMP Quick Start Guide
- Next Generation Pumps: Best Practices Before Transfection
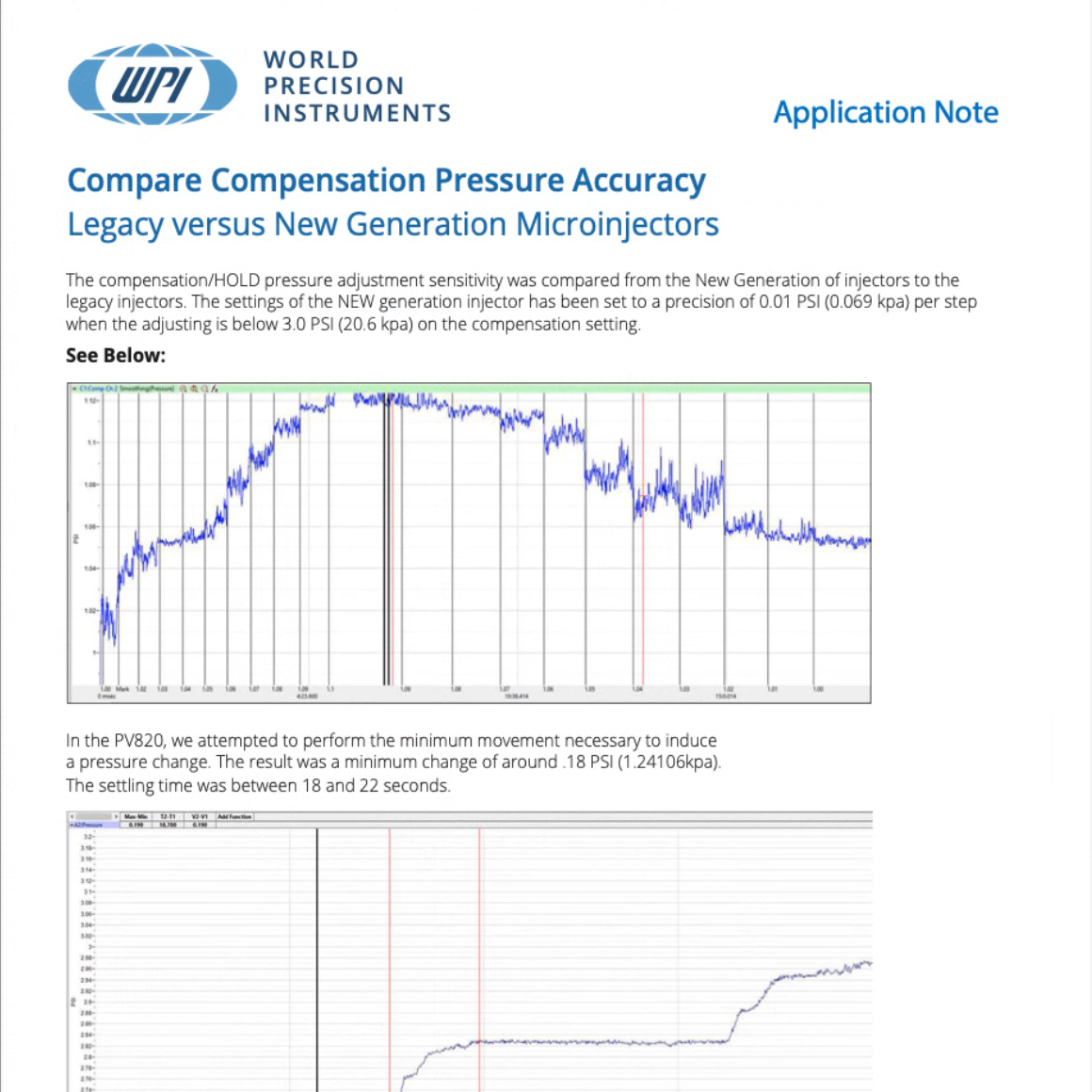
Compare the compensation pressure accuracy of the older generation legacy pump (PV820) with the new generation of pressure microinjectors (PV850 used in this example).
Videos
WPI's New MICRO-ePUMP Microinjector with Integrated Pressure Source
WPI's New PV850 Microinjector: Setting Up the Hardware
WPI's New PV850 Microinjector: Using the Software
PV850 Microinjector Setup Tip
µPUMP tips
MICRO-ePUMP Specifications