This website uses cookies to ensure you get the best experience on our website.
Read more
WPI Manual Micromanipulator
As low as
$1,531.00
Only %1 left
Prices valid in USA, Canada, and PR only.
Order code
Price range: $1,531 - $1,759

Prices valid in USA, Canada, and PR only.
The M3301 is a popular Manual Micromanipulator, because it is accurate, lightweight and well-engineered. This solid manipulator outsells all others worldwide for high precision experiments where magnification is in the range of up to 250x.
Weighing just 550g, it has a slim, space-saving design. Units can stand tightly grouped, since all control knobs project to the rear. Because the control knobs are clustered within an 8 cm area in a single vertical plane, resolution is quick. The hand works blindly while the eye monitors the microscopic image. Vernier scales allow readings to 0.1 mm. X-axis fine control allows readings to 10μm.
The rack-and-pinion drive, V-shaped guideways and cross roller bearings ensure smooth movement that is sure and repeatable, without drift, side play, backlash or sticking. Contact parts are milled of hardened steel for high performance and long life.
Prices valid in USA, Canada, and PR only.
Popular manual micromanipulator
- The most widely used micromanipulator
- Lightweight (550 g)
- Sure, repeatable movement without drift
- Choice of optional M-3 tilting base
- Thumbscrew included for metric anti-vibration platforms with M6 holes
- 5464 Base Weight and 15873 Angled Electrode Holder sold separately
- Comes installed with a 12 mm clamp for the M10 magnetic base
Solid Micromanipulator Design for Smooth, Repeatable Movements
The M3301 is a popular Manual Micromanipulator, because it is accurate, lightweight and well-engineered. This solid manipulator outsells all others worldwide for high precision experiments where magnification is in the range of up to 250x.
Weighing just 550g, it has a slim, space-saving design. Units can stand tightly grouped, since all control knobs project to the rear. Because the control knobs are clustered within an 8 cm area in a single vertical plane, resolution is quick. The hand works blindly while the eye monitors the microscopic image. Vernier scales allow readings to 0.1 mm. X-axis fine control allows readings to 10μm.
The rack-and-pinion drive, V-shaped guideways and cross roller bearings ensure smooth movement that is sure and repeatable, without drift, side play, backlash or sticking. Contact parts are milled of hardened steel for high performance and long life.
Benefits
- Easy access controls. All control knobs are clustered in an 8 cm area in a single vertical plane for quick resolution, allowing you to focus and easily manipulate the controls without looking away from the microscope.
- Lightweight and portable, requiring little bench space
- Flexible mounting options. Use a tilt base, a ring clamp, or mount directly to air table.
- Smooth, repeatable movements. Rack-and-pinion drive, V-shaped guideways and cross roller bearings ensure smooth movement without drift, side play, backlash or sticking.
- Long life. Hardened steel components ensure high performance for the long haul.
Applications
- Microinjection
- Electrophysiology recording
Important Notes
Left- or right-handed versions of the M3301 are supplied with:
When it is sold with the M-3 magnetic stand, the M3301-M3 base is 1.25" tall.
Options
| Order code | Description |
| M3301R | M3301 Micromanipulator Right hand |
| M3301L | M3301 Micromanipulator Left hand |
| M3301-M3-R | M3301 Micromanipulator M3 Tilting Base Right hand 5# weight not included |
| M3301-M3-L | M3301 Micromanipulator M3 Tilting Base Left hand 5# weight not included |
Benefits
- Control knobs clustered in 8 cm area in a single vertical plane for quick resolution
- Right and left had orientation options available
Applications
- Microinjection
- Electrophysiology recording
Weighing just 550g and employing a slim space-saving design, this well-built manipulator outsells all others worldwide for high precision experiments where magnification is in the range of up to 250x. Its design allows units to stand tightly grouped - since all control knobs project to the rear. Because the control knobs are clustered within an 8 cm area in a single vertical plane, resolution is quick. The hand works blindly while the eye monitors the microscopic image. Vernier scales allow readings to 0.1 mm. X-axis fine control allows readings to 10μm.
The instrument employs rack-and-pinion drive, V-shaped guideways and cross roller bearings, so all movement is sure and repeatable, without drift, side play, backlash or sticking. Contact parts are milled of hardened steel for high performance and long life.
Left- or right-handed versions of the M3301 are supplied with:
With the M-3 magnetic stand, the M3301-M3 base is 1.25" tall.
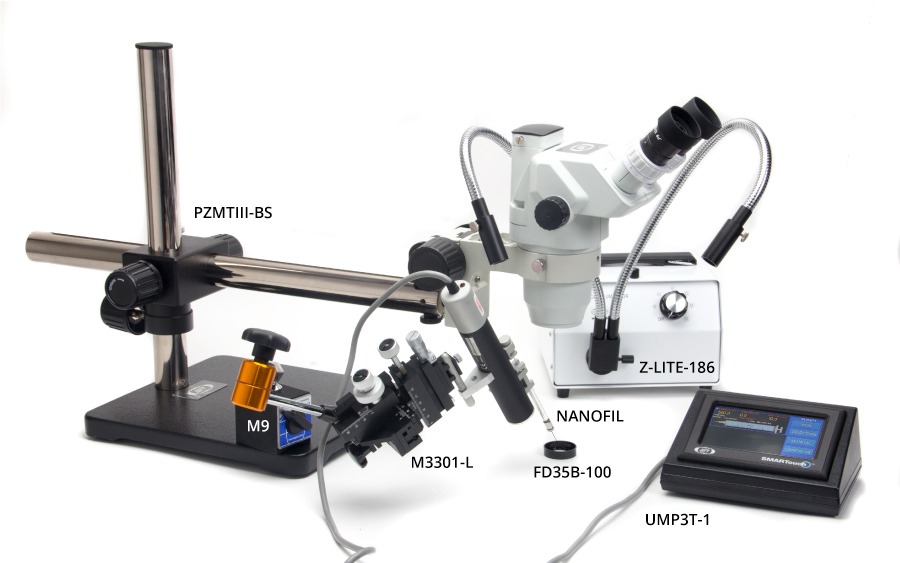
The following images show various setups for microinjection. Keep in mind that parts are interchangeable. For example:
- M10 or the M9 magnetic base could be used.
- PZMIV microscope could be used instead of the PZMIII.
- M3301 or the KITE micromanipulators can be used, and these micromanipulators can be placed on either side. (Keep in mind, though, If you wanted to use a KITE on the right side of the setup below, you would order a KITE-R (right hand), or if you want an M3301 on the left side, you would order an M3301.)
- 5479 or 5052 magnetic bases are virtually interchangeable.
- One or two Nanoliters, one or two UMPIII systems, or one Nanoliter and one UMPIII may be used, as desired.
ONE NANOLITER/ONE UMP3-1

ONE NANOLITER

ONE NANOLITER/M3301

ONE NANOLITER/KITE

ONE UMP3-1
.
| SKU | VAR-3093 |
|---|
Upsell Products
Manipulator Care Instruction Sheet
Video
Mounting Microelectrode Holder on M3301 Micromanipulator
Tips for Using Your Manual Micromanipulator
Mounting M3301 Micromanipulator on Tilt Base
Adjusting for Mechanical Drift in a KITE Manual Micromanipulator
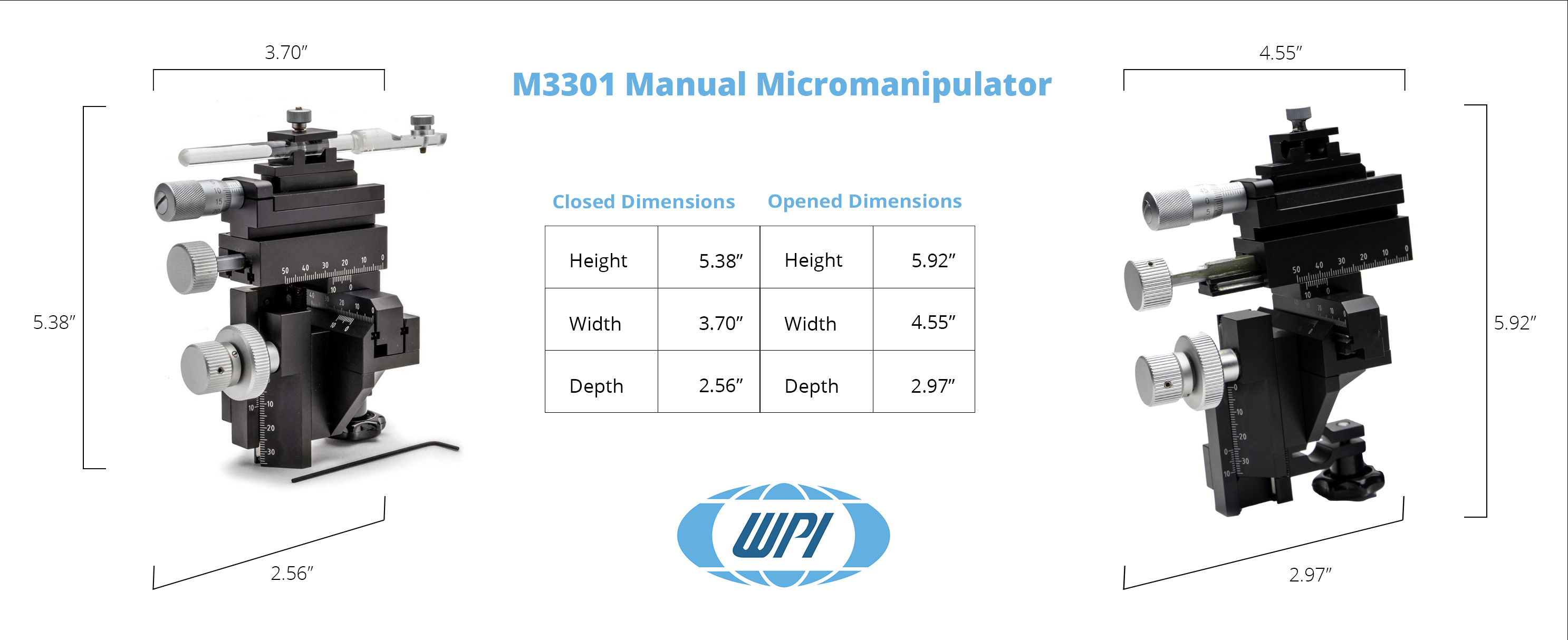
| Travel Range | Resolution | |
| X-axis Fine | 10 mm | 0.01 mm |
| X-axis | 37 mm | 0.1 mm |
| Y-axis | 20 mm | 0.1 mm |
| Z-axis | 25 mm | 0.1 mm |
| Shipping Weight | 3 lbs (1.4 kg) |
Whole-embryo culture of mouse embryos to study vascular development. (n.d.). Retrieved October 23, 2015, from http://docserv.uni-duesseldorf.de/servlets/DerivateServlet/Derivate-26166/PhDThesis_MartinZeeb.pdf
Evsen, L., & Doetzlhofer, A. (2016). Gene Transfer into the Chicken Auditory Organ by <em>In Ovo</em> Micro-electroporation. Journal of Visualized Experiments, (110), e53864–e53864. http://doi.org/10.3791/53864
Grossöhmichen, M., Salcher, R., Püschel, K., Lenarz, T., & Maier, H. (2016). Differential Intracochlear Sound Pressure Measurements in Human Temporal Bones with an Off-the-Shelf Sensor. BioMed Research International, 2016, 6059479. http://doi.org/10.1155/2016/6059479
Ito, Y. A., Belforte, N., Cueva Vargas, J. L., & Di Polo, A. (2016). A Magnetic Microbead Occlusion Model to Induce Ocular Hypertension-Dependent Glaucoma in Mice. Journal of Visualized Experiments, (109), e53731–e53731. http://doi.org/10.3791/53731
Grossöhmichen, M., Salcher, R., Kreipe, H.-H., Lenarz, T., & Maier, H. (2015). The CodacsTM Direct Acoustic Cochlear Implant Actuator: Exploring Alternative Stimulation Sites and Their Stimulation Efficiency. PLOS ONE, 10(3), e0119601. http://doi.org/10.1371/journal.pone.0119601
Mobberley, J. M., Khodadad, C. L. M., Visscher, P. T., Reid, R. P., Hagan, P., & Foster, J. S. (2015). Inner workings of thrombolites: spatial gradients of metabolic activity as revealed by metatranscriptome profiling. Scientific Reports, 5, 12601. http://doi.org/10.1038/srep12601
Nesbit, S. C., Van Hoof, A. G., Le, C. C., Dearworth, J. R., & Jr. (2015). Extracellular Recording of Light Responses from Optic Nerve Fibers and the Caudal Photoreceptor in the Crayfish. Journal of Undergraduate Neuroscience Education : JUNE : A Publication of FUN, Faculty for Undergraduate Neuroscience, 14(1), A29-38. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/26557793
Bouta, E. M., Wood, R. W., Brown, E. B., Rahimi, H., Ritchlin, C. T., & Schwarz, E. M. (2014). In vivo quantification of lymph viscosity and pressure in lymphatic vessels and draining lymph nodes of arthritic joints in mice. The Journal of Physiology, 592(6), 1213–1223. http://doi.org/10.1113/jphysiol.2013.266700
Cornwall, C. E., Boyd, P. W., McGraw, C. M., Hepburn, C. D., Pilditch, C. A., Morris, J. N., … Hurd, C. L. (2014). Diffusion boundary layers ameliorate the negative effects of ocean acidification on the temperate coralline macroalga Arthrocardia corymbosa. PloS One, 9(5), e97235. http://doi.org/10.1371/journal.pone.0097235
Shin, S.-H., Lee, S., Bae, J.-S., Jee, J.-G., Cha, H.-J., & Lee, Y. M. (2014). Thymosin beta4 regulates cardiac valve formation via endothelial-mesenchymal transformation in zebrafish embryos. Molecules and Cells, 37(4), 330–6. http://doi.org/10.14348/molcells.2014.0003
Gharbaran, R., & Aisemberg, G. O. (2013). Identification of leech embryonic neurons that express a Hox gene required for the differentiation of a paired, segment-specific motor neuron. International Journal of Developmental Neuroscience : The Official Journal of the International Society for Developmental Neuroscience, 31(2), 105–15. http://doi.org/10.1016/j.ijdevneu.2012.11.004
Loch, D., Heidel, C., Breer, H., & Strotmann, J. (2013). Adiponectin Enhances the Responsiveness of the Olfactory System. PLoS ONE, 8(10), e75716. http://doi.org/10.1371/journal.pone.0075716
Luetje, C. W., Nichols, A. S., Castro, A., & Sherman, B. L. (2013). Functional assay of mammalian and insect olfactory receptors using Xenopus oocytes. Methods in Molecular Biology (Clifton, N.J.), 1003, 187–202. http://doi.org/10.1007/978-1-62703-377-0_14
Lyons-Warren, A. M., Kohashi, T., Mennerick, S., & Carlson, B. A. (2013). Retrograde Fluorescent Labeling Allows for Targeted Extracellular Single-unit Recording from Identified Neurons <em>In vivo</em> Journal of Visualized Experiments, (76), e3921–e3921. http://doi.org/10.3791/3921
Saha, D., Leong, K., Katta, N., & Raman, B. (2013). Multi-unit Recording Methods to Characterize Neural Activity in the Locust (<em>Schistocerca Americana</em>) Olfactory Circuits. Journal of Visualized Experiments, (71), e50139–e50139. http://doi.org/10.3791/50139
Saha, D., Leong, K., Katta, N., & Raman, B. (2013). Multi-unit recording methods to characterize neural activity in the locust (Schistocerca americana) olfactory circuits. Journal of Visualized Experiments : JoVE, (71). http://doi.org/10.3791/50139
Spencer, N. J. (2013). Characteristics of colonic migrating motor complexes in neuronal NOS (nNOS) knockout mice. Frontiers in Neuroscience, 7, 184. http://doi.org/10.3389/fnins.2013.00184
Bryant, L. D., Little, J. C., & Bürgmann, H. (2012). Response of sediment microbial community structure in a freshwater reservoir to manipulations in oxygen availability. FEMS Microbiology Ecology, 80(1), 248–63. http://doi.org/10.1111/j.1574-6941.2011.01290.x
Chernet, B. T., Adams, D. S., & Levin, M. (2012). Photoconversion for tracking the dynamics of cell movement in Xenopus laevis embryos. Cold Spring Harbor Protocols, 2012(6), 683–90. http://doi.org/10.1101/pdb.prot068502
Chernet, B. T., & Levin, M. (2012). A versatile protocol for mRNA electroporation of Xenopus laevis embryos. Cold Spring Harbor Protocols, 2012(4), 447–52. http://doi.org/10.1101/pdb.prot067694
Laude, N. D., Atcherley, C. W., & Heien, M. L. (2012). Rethinking data collection and signal processing. 1. Real-time oversampling filter for chemical measurements. Analytical Chemistry, 84(19), 8422–6. http://doi.org/10.1021/ac302169y
Wang, Y., Shah, P., Phillips, C., Sims, C. E., & Allbritton, N. L. (2012). Trapping cells on a stretchable microwell array for single-cell analysis. Analytical and Bioanalytical Chemistry, 402(3), 1065–72. http://doi.org/10.1007/s00216-011-5535-9
Zeeb, M., Axnick, J., Planas-Paz, L., Hartmann, T., Strilic, B., & Lammert, E. (2012). Pharmacological manipulation of blood and lymphatic vascularization in ex vivo-cultured mouse embryos. Nature Protocols, 7(11), 1970–82. http://doi.org/10.1038/nprot.2012.120
Dong, Z., Wagle, M., & Guo, S. (2011). Time-lapse live imaging of clonally related neural progenitor cells in the developing zebrafish forebrain. Journal of Visualized Experiments : JoVE, (50). http://doi.org/10.3791/2594
Allen, M. J., & Godenschwege, T. A. (2010). Electrophysiological recordings from the Drosophila giant fiber system (GFS). Cold Spring Harbor Protocols, 2010(7), pdb.prot5453. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2946074&tool=pmcentrez&rendertype=abstract
Cianciolo Cosentino, C., Roman, B. L., Drummond, I. A., & Hukriede, N. A. (2010). Intravenous Microinjections of Zebrafish Larvae to Study Acute Kidney Injury. Journal of Visualized Experiments, (42), e2079–e2079. http://doi.org/10.3791/2079
Cygnar, K. D., Stephan, A. B., & Zhao, H. (2010). Analyzing Responses of Mouse Olfactory Sensory Neurons Using the Air-phase Electroolfactogram Recording. Journal of Visualized Experiments, (37), e1850–e1850. http://doi.org/10.3791/1850
Kulesa, P. M., Teddy, J. M., Smith, M., Alexander, R., Cooper, C. H., Lansford, R., & McLennan, R. (2010). Multispectral fingerprinting for improved in vivo cell dynamics analysis. BMC Developmental Biology, 10(1), 101. http://doi.org/10.1186/1471-213X-10-101
Seidl, A. H., & Rubel, E. W. (2010). A simple method for multiday imaging of slice cultures. Microscopy Research and Technique, 73(1), 37–44. http://doi.org/10.1002/jemt.20750
Kasri, N. N., Govek, E.-E., & Van Aelst, L. (2008). Characterization of oligophrenin-1, a RhoGAP lost in patients affected with mental retardation: lentiviral injection in organotypic brain slice cultures. Methods in Enzymology, 439, 255–66. http://doi.org/10.1016/S0076-6879(07)00419-3
Kasri, N. N., Govek, E.-E., & Van Aelst, L. (2008). Small GTPases in Disease, Part B. Methods in enzymology (Vol. 439). Elsevier. http://doi.org/10.1016/S0076-6879(07)00419-3
Rumpler, M., Woesz, A., Dunlop, J. W. ., van Dongen, J. T., & Fratzl, P. (2008). The effect of geometry on three-dimensional tissue growth. Journal of The Royal Society Interface, 5(27), 1173–1180. http://doi.org/10.1098/rsif.2008.0064
Fleisch, V. C., Jametti, T., & Neuhauss, S. C. F. (2008). Electroretinogram (ERG) Measurements in Larval Zebrafish. CSH Protocols, 2008(3), pdb.prot4973. http://doi.org/10.1101/PDB.PROT4973
Kasemeier-Kulesa, J. C., Bradley, R., Pasquale, E. B., Lefcort, F., & Kulesa, P. M. (2006). Eph/ephrins and N-cadherin coordinate to control the pattern of sympathetic ganglia. Development (Cambridge, England), 133(24), 4839–47. http://doi.org/10.1242/dev.02662
Spencer, N. J., & Smith, T. K. (2004). Mechanosensory S-neurons rather than AH-neurons appear to generate a rhythmic motor pattern in guinea-pig distal colon. The Journal of Physiology, 558(2), 577–596. http://doi.org/10.1113/jphysiol.2004.063586
Padnick, L. B., & Linsenmeier, R. A. (1999). Properties of the flash visual evoked potential recorded in the cat primary visual cortex. Vision Research, 39(17), 2833–40. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10492813
Oemar, B. S., Tschudi, M. R., Godoy, N., Brovkovich, V., Malinski, T., & Lüscher, T. F. (1998). Reduced Endothelial Nitric Oxide Synthase Expression and Production in Human Atherosclerosis. Circulation, 97(25).