This website uses cookies to ensure you get the best experience on our website.
Read more
Constant Current Stimulus Isolator
As low as
$1,698.00
Only %1 left
Prices valid in USA, Canada, and PR only.
Order code
Price range: $1,698 - $2,438

Prices valid in USA, Canada, and PR only.
Activated by conventional logic-level commands, Model A365 can be gated by any pulse generator, stimulator or computer output with automated bipolar pulsing for zero net charge on biological preparations.
To learn more about our warranty options, click here.
Prices valid in USA, Canada, and PR only.
Automated bipolar pulsing for zero net charge on biological preparations
Features
- Constant current
- Unipolar and bipolar stimulation modes
- Built-in non-compliance alarm
- Input is optically isolated
- Standard TTL triggering
- DC test mode
- Powered by 9 V alkaline or rechargeable batteries
Options
| Part # | Description | Battery Type | Includes Charger |
| A365RC | A365R with an A362 Battery Charger | Rechargeable Battery | Yes |
| SYS-A365R | High Voltage Isolator, Bipolar | Rechargeable Battery | No |
| SYS-A365D | High Voltage Isolator, Bipolar | Alkaline Batteries | _ |
Benefits
- Compliance voltage is 100V or better
- Bipolar mode auto generates alternating positive and negative pulses from TTL input
- Test mode simplifies performance verification
- Optical isolation enhances safety of the preparation and reduces noise susceptibility
- Premium Warranty Available
Applications
- Electrophysiology
- Brain slice stimulation
- In vivo brain and CNS stimulation
Activated by conventional logic-level commands, Model A365 can be gated by any pulse generator, stimulator or computer output with automated bipolar pulsing for zero net charge on biological preparations.
Dual tone audible alarms
A tone sounds when an open electrode circuit is detected or when system compliance is reached. A second optional tone sounds when a signal is applied to the input. A test switch is also provided to check battery charge.
Current delivery up to 10 mA at more than 100V
Stimulus currents are set using a three-digit control knob and a three-position range switch. Output current tracks control settings to better than 1%. Output current is load independent, and voltage sufficient to push the desired current through the load is automatically developed, subject only to compliance limits. Model A360LA produces up to 10 mA current, in three ranges, at more than 100 V compliance.
Bipolar Output Polarity
Output polarity is determined by a push switch on the front panel. Bipolar current is toggled by the command waveform, setting alternating pulses as positive or negative.
Power
This A365RC Stimulus Isolator includes both the A365R Stimulus Isolator and the A362 battery charger.The rechargeable A365R is supplied with a nickel metal hydride battery stack. The A362 Battery Charger is required with the A365R.
NOTE: Not intended for human use.
| SKU | VAR-2273 |
|---|
Upsell Products
-
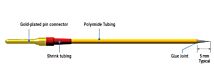
Tungsten Profile C, 127 mm long
As low as $199.00
| OUTPUT WAVEFORM | DC or current pulse |
| OUTPUT CURRENT RANGES | 0.1, 1.0, and 10 mA |
| CURRENT AMPLITUDE ERROR | 0.5% of full scale, max. |
| CURRENT RESOLUTION | 0.1% of full scale, typical |
| OUTPUT LOAD VOLTAGE EXCURSION (COMPLIANCE) | 100 V |
| EXTERNAL COMMAND THRESHOLD | 5 V at 3 mA, min. 10 V, max. |
| TRIGGER THRESHOLD | 2.0 V at 0.5 mA |
| OUTPUT POLARITY | Reversible, manual switch or automatic |
| CURRENT RISE TIME & DELAY | 6 μs, typical (1 KΩ load) |
| CURRENT FALL TIME & DELAY | 10 μs, typical (1 KΩ load) |
| OUTPUT TO GROUND RESISTANCE | 1012 Ω |
| OPTOCOUPLER | 2500 V, rated min. breakdown voltage |
| POWER: Model A365D (dry cell) | 16 alkaline 9 V batteries, included |
| POWER: Model A365R (rechargeable) | 16 rechargeable NiMH 9 V batteries included |
| DIMENSIONS | 8.5 x 3.5 x 5 in. (22 x 9 x 12 cm) |
| SHIPPING WEIGHT | 4 lb. (1.8 kg) |
Yavich, L., Tanila, H., Vepsäläinen, S., & Jäkälä, P. (n.d.). Neurobiology of Disease Role of ␣-Synuclein in Presynaptic Dopamine Recruitment. https://doi.org/10.1523/JNEUROSCI.2559-04.2004
Iremonger, K. J., Anderson, T. R., Hu, B., & Kiss, Z. H. T. (n.d.). Cellular Mechanisms Preventing Sustained Activation of Cortex During Subcortical High-Frequency Stimulation. https://doi.org/10.1152/jn.00105.2006
Rowland, N. C., & Jaeger, D. (n.d.). Responses to Tactile Stimulation in Deep Cerebellar Nucleus Neurons Result From Recurrent Activation in Multiple Pathways. https://doi.org/10.1152/jn.01100.2007
D ’ambrosio, R., Gordon, D. S., Winn, H. R., ’ambrosio, D., Raimondo, D. S., Gordon, H., & Richard, W. (n.d.). Differential Role of KIR Channel and Na ϩ /K ϩ -Pump in the Regulation of Extracellular K ϩ in Rat Hippocampus. https://doi.org/10.1152/jn.00240.2001
Huda, R., Mccrimmon, D. R., & Martina, M. (n.d.). pH modulation of glial glutamate transporters regulates synaptic transmission in the nucleus of the solitary tract.
Chen, Y., Beffert, U., Ertunc, M., Tang, T.-S., Kavalali, E. T., Bezprozvanny, I., & Herz, J. (n.d.). Development/Plasticity/Repair Reelin Modulates NMDA Receptor Activity in Cortical Neurons. https://doi.org/10.1523/JNEUROSCI.1951-05.2005
D ’ambrosio, R., Wenzel, J., Schwartzkroin, P. A., Mckhann Ii, G. M., & Janigro, D. (n.d.). Functional Specialization and Topographic Segregation of Hippocampal Astrocytes.
D ’ambrosio, R., Maris, D. O., Grady, M. S., Winn, H. R., & Janigro, D. (n.d.). Impaired K ؉ Homeostasis and Altered Electrophysiological Properties of Post-Traumatic Hippocampal Glia.
Ji, H., & Shepard, P. D. (n.d.). Behavioral/Systems/Cognitive Lateral Habenula Stimulation Inhibits Rat Midbrain Dopamine Neurons through a GABA A Receptor-Mediated Mechanism. https://doi.org/10.1523/JNEUROSCI.0958-07.2007
Lee, E., Hong, J., Park, Y.-G., Chae, S., Kim, Y., & Kim, D. (2015). Left brain cortical activity modulates stress effects on social behavior. Scientific Reports, 5, 13342. https://doi.org/10.1038/srep13342
Gindrat, A.-D., Quairiaux, C., Britz, J., Brunet, D., Lanz, F., Michel, C. M., & Rouiller, E. M. (2015). Whole-scalp EEG mapping of somatosensory evoked potentials in macaque monkeys. Brain Structure & Function, 220(4), 2121–2142. https://doi.org/10.1007/s00429-014-0776-y
Avila, I., & Lin, S.-C. (2014). Motivational Salience Signal in the Basal Forebrain Is Coupled with Faster and More Precise Decision Speed. PLoS Biology, 12(3), e1001811. https://doi.org/10.1371/journal.pbio.1001811
Nguyen, D. P., & Lin, S.-C. (2014). A frontal cortex event-related potential driven by the basal forebrain. ELife, 3, e02148. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3974155&tool=pmcentrez&rendertype=abstract
Herrera, C., Directores, R., Panetsos, F., Carlos, P., & Trueba, A. (2014). TESIS DOCTORAL Efectos de la estimulación artificial de un nervio periférico seccionado sobre la vía somatosensorial desaferentizada de la rata.
Younce, J. R., Albaugh, D. L., & Shih, Y.-Y. I. (2014). Deep Brain Stimulation with Simultaneous fMRI in Rodents. Journal of Visualized Experiments, (84), e51271–e51271. https://doi.org/10.3791/51271
Oulad Ben Taib, N., & Manto, M. (2013). Trains of Epidural DC Stimulation of the Cerebellum Tune Corticomotor Excitability. Neural Plasticity, 2013(10), 1–12. https://doi.org/10.1155/2013/613197
Syvänen, S., Russmann, V., Verbeek, J., Eriksson, J., Labots, M., Zellinger, C., … Potschka, H. (2013). [11C]quinidine and [11C]laniquidar PET imaging in a chronic rodent epilepsy model: Impact of epilepsy and drug-responsiveness. Nuclear Medicine and Biology, 40(6), 764–775. https://doi.org/10.1016/j.nucmedbio.2013.05.008
Schroder, E. A., Lefta, M., Zhang, X., Bartos, D. C., Feng, H.-Z., Zhao, Y., … Delisle, B. P. (2013). The cardiomyocyte molecular clock, regulation of Scn5a, and arrhythmia susceptibility. American Journal of Physiology. Cell Physiology, 304(10), C954-65. https://doi.org/10.1152/ajpcell.00383.2012
Schmuckermair, C., Gaburro, S., Sah, A., Landgraf, R., Sartori, S. B., & Singewald, N. (2013). Behavioral and neurobiological effects of deep brain stimulation in a mouse model of high anxiety- and depression-like behavior. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 38(7), 1234–1244. https://doi.org/10.1038/npp.2013.21
Dalby-Brown, W., Jessen, C., Hougaard, C., Jensen, M. L., Jacobsen, T. A., Nielsen, K. S., … Jørgensen, S. (2013). Characterization of a novel high-potency positive modulator of Kv7 channels. European Journal of Pharmacology, 709(1–3), 52–63. https://doi.org/10.1016/j.ejphar.2013.03.039
Licko, T., Seeger, N., Zellinger, C., Russmann, V., Matagne, A., & Potschka, H. (2013). Lacosamide treatment following status epilepticus attenuates neuronal cell loss and alterations in hippocampal neurogenesis in a rat electrical status epilepticus model. Epilepsia, 54(7), 1176–1185. https://doi.org/10.1111/epi.12196
Atherton, J. F., Menard, A., Urbain, N., & Bevan, M. D. (2013). Short-term depression of external globus pallidus-subthalamic nucleus synaptic transmission and implications for patterning subthalamic activity. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 33(17), 7130–7144. https://doi.org/10.1523/JNEUROSCI.3576-12.2013
Saha, D., Leong, K., Katta, N., & Raman, B. (2013). Multi-unit Recording Methods to Characterize Neural Activity in the Locust (<em>Schistocerca Americana</em>) Olfactory Circuits. Journal of Visualized Experiments, (71), e50139–e50139. https://doi.org/10.3791/50139
Huda, R., McCrimmon, D. R., & Martina, M. (2013). pH modulation of glial glutamate transporters regulates synaptic transmission in the nucleus of the solitary tract. Journal of Neurophysiology, 110(2), 368–377. https://doi.org/10.1152/jn.01074.2012
Sonner, P. M., & Ladle, D. R. (2013). Early postnatal development of GABAergic presynaptic inhibition of Ia proprioceptive afferent connections in mouse spinal cord. Journal of Neurophysiology, 109(8), 2118–2128. https://doi.org/10.1152/jn.00783.2012
Zhu, Z., Sierra, A., Burnett, C. M.-L. L., Chen, B., Subbotina, E., Koganti, S. R. K., … Zingman, L. V. (2013). Sarcolemmal ATP-sensitive potassium channels modulate skeletal muscle function under low-intensity workloads. The Journal of General Physiology, 143(1), 119–134. https://doi.org/10.1085/jgp.201311063
Brenowitz, S. D., & Regehr, W. G. (2012). Presynaptic imaging of projection fibers by in vivo injection of dextran-conjugated calcium indicators. Cold Spring Harbor Protocols, 2012(4), 465–471. https://doi.org/10.1101/pdb.prot068551
Li, W., Janardhan, A. H., Fedorov, V. V, Sha, Q., Schuessler, R. B., & Efimov, I. R. (2011). Low-energy multistage atrial defibrillation therapy terminates atrial fibrillation with less energy than a single shock. Circulation. Arrhythmia and Electrophysiology, 4(6), 917–925. https://doi.org/10.1161/CIRCEP.111.965830
Mathis, D. M., Furman, J. L., & Norris, C. M. (2011). Preparation of Acute Hippocampal Slices from Rats and Transgenic Mice for the Study of Synaptic Alterations during Aging and Amyloid Pathology. Journal of Visualized Experiments, (49), e2330–e2330. https://doi.org/10.3791/2330
Manto, M. U., Hampe, C. S., Rogemond, V., & Honnorat, J. (2011). Respective implications of glutamate decarboxylase antibodies in stiff person syndrome and cerebellar ataxia. Orphanet Journal of Rare Diseases, 6(1), 3. https://doi.org/10.1186/1750-1172-6-3
Kim, J., Woo, J., Park, Y.-G., Chae, S., Jo, S., Choi, J. W., … Kim, D. (2011). Thalamic T-type Ca2+ channels mediate frontal lobe dysfunctions caused by a hypoxia-like damage in the prefrontal cortex. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 31(11), 4063–4073. https://doi.org/10.1523/JNEUROSCI.4493-10.2011
Seo, J. H., Jang, I. K., Kim, H., Yang, M. S., Lee, J. E., Kim, H. E., … Cho, S.-R. (2011). Early Immunomodulation by Intravenously Transplanted Mesenchymal Stem Cells Promotes Functional Recovery in Spinal Cord Injured Rats. Cell Medicine, 2(2), 55–67. https://doi.org/10.3727/215517911X582788
Pelkonen, A., Hiltunen, M., Kiianmaa, K., & Yavich, L. (2010). Stimulated dopamine overflow and alpha-synuclein expression in the nucleus accumbens core distinguish rats bred for differential ethanol preference. Journal of Neurochemistry, 114(4), 1168–1176. https://doi.org/10.1111/j.1471-4159.2010.06844.x
Foust, A. J., Schei, J. L., Rojas, M. J., & Rector, D. M. (2008). In vitro and in vivo noise analysis for optical neural recording. Journal of Biomedical Optics, 13(4), 044038. https://doi.org/10.1117/1.2952295
Schei, J. L., McCluskey, M. D., Foust, A. J., Yao, X.-C., & Rector, D. M. (2008). Action potential propagation imaged with high temporal resolution near-infrared video microscopy and polarized light. NeuroImage, 40(3), 1034–1043. https://doi.org/10.1016/j.neuroimage.2007.12.055
Foust, A. J., & Rector, D. M. (2007). Optically teasing apart neural swelling and depolarization. Neuroscience, 145(3), 887–899. https://doi.org/10.1016/j.neuroscience.2006.12.068
Ji, H., & Shepard, P. D. (2007). Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor-mediated mechanism. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 27(26), 6923–6930. https://doi.org/10.1523/JNEUROSCI.0958-07.2007
Lee, B. H., Lee, K. H., Yoon, D. H., Kim, U. J., Hwang, Y. S., Park, S. K., … Jahng, T. (2005). Effects of methylprednisolone on the neural conduction of the motor evoked potentials in spinal cord injured rats. Journal of Korean Medical Science, 20(1), 132–138. https://doi.org/10.3346/jkms.2005.20.1.132
Anderson, T., Hu, B., Pittman, Q., & Kiss, Z. H. T. (2004). Mechanisms of deep brain stimulation: an intracellular study in rat thalamus. The Journal of Physiology, 559(1), 301–313. https://doi.org/10.1113/jphysiol.2004.064998
Sokolow, S., Manto, M., Gailly, P., Molgó, J., Vandebrouck, C., Vanderwinden, J.-M., … Schurmans, S. (2004). Impaired neuromuscular transmission and skeletal muscle fiber necrosis in mice lacking Na/Ca exchanger 3. Journal of Clinical Investigation, 113(2), 265–273. https://doi.org/10.1172/JCI18688
D’Ambrosio, R., Fairbanks, J. P., Fender, J. S., Born, D. E., Doyle, D. L., & Miller, J. W. (2004). Post-traumatic epilepsy following fluid percussion injury in the rat. Brain : A Journal of Neurology, 127(Pt 2), 304–314. https://doi.org/10.1093/brain/awh038
Shaw, B. K., & Kennedy, G. G. (2002). Evidence for species differences in the pattern of androgen receptor distribution in relation to species differences in an androgen-dependent behavior. Journal of Neurobiology, 52(3), 203–220. https://doi.org/10.1002/neu.10079
Yavich, L., & Tiihonen, J. (2000). Ethanol modulates evoked dopamine release in mouse nucleus accumbens: dependence on social stress and dose. European Journal of Pharmacology, 401(3), 365–373. Retrieved from http://www.safetylit.org/citations/index.php?fuseaction=citations.viewdetails&citationIds%5B%5D=citjournalarticle_271069_38
Knisley, S. B., Trayanova, N., & Aguel, F. (1999). Roles of Electric Field and Fiber Structure in Cardiac Electric Stimulation. Biophysical Journal, 77(3), 1404–1417. https://doi.org/10.1016/S0006-3495(99)76989-4