This website uses cookies to ensure you get the best experience on our website.
Read more
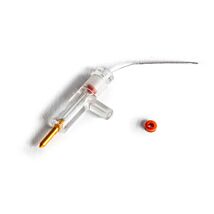
HOLDER & 3 HYDROGEN ELECTRODES
$406.00
Prices valid in USA, Canada, and PR only.
Order code
KWIKH-2

Prices valid in USA, Canada, and PR only.
Accurately measure hydrogen
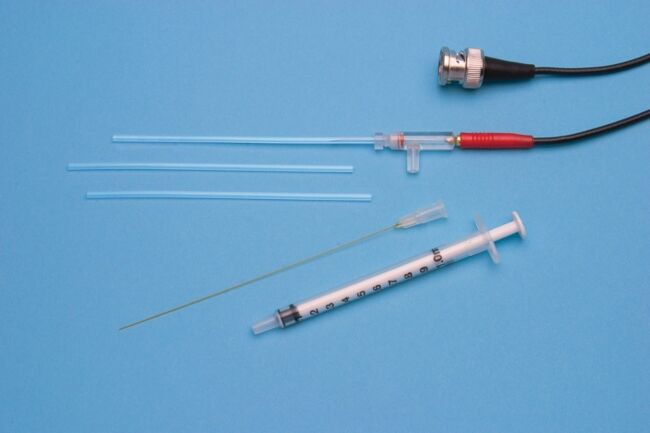
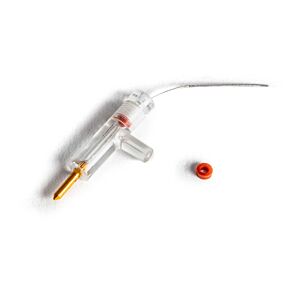
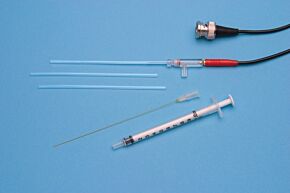
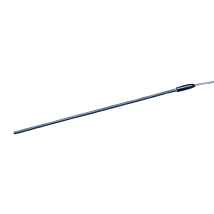
These highly stable electrodes accurately measure calcium, potassium, hydrogen and TPP (Tetraphenylphosphonium) ion activity. Tips consist of 2 mm diameter plastic tubes sealed at one end with an ion-sensitive membrane. After filling with electrolyte solution, you can insert the tube into the holder and connect it to a pH meter. Tips and holders are interchangeable, so one tip may be replaced with another sensitive to a different ion. Replacing a tip takes less than a minute. Electrode tips normally last several months, when stored properly in saline solution. When replacement is necessary, only the tip needs be replaced.
Prices valid in USA, Canada, and PR only.
Features
- Fast, accurate, economical
- Superior, stable PVC membrane
- Fast response
- 2mm diameter tips
- Interchangeable tip holder
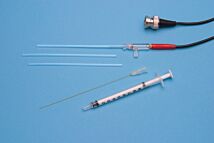
- Each kit includes 3 electrode tips and MicroFil filling syringe
Benefits
- Inexpensive
- Use to measure various ions in biological media
Applications
- Detection of ions in vivo or in vitro for biological applications
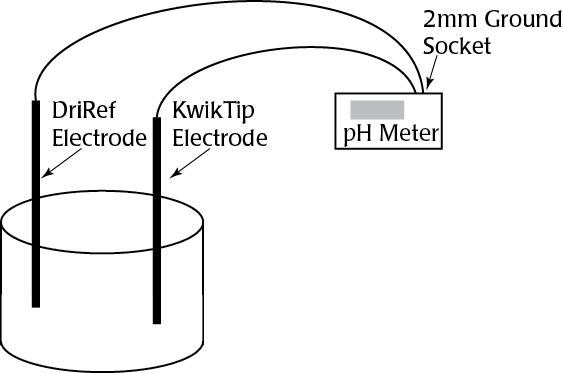
Kwik-Tip electrodes are available separately and as kits. Each “KWIK” Electrode Holder kit includes a reusable holder and three removable tips. In addition to a 4-foot BNC cable and an electrolyte filling syringe; “TIP” Electrode Kits contain three electrode tips for a specific ion. A separate reference electrode, such as WPI’s Dri-Ref™, is also required.
If your pH meter requires a US Standard connector, you will also need Part 3508 (BNC-to-US pH Standard adapter).
This product requires the use of a reference electrode, like the DriRef-2. For a complete list of the DriRef electrodes, refer to the Accessories tab.
| SKU | KWIKH-2 |
|---|
Upsell Products
| Electrode |
Color Code |
Filling Solution |
Min. Slope/Decade |
Concentration Range |
Selectivity Coeffiecients (-log) |
|
| TIPCA |
Calcium | Green | 0.1M CaCl2 | 28mV | 0.1 - 10-6.75M | Na+ 5.5, K+ 5.4, Mg++ 4.9 |
| TIPH |
Hydrogen | Orange | 1M Citric acid, 0.01M NaCl, pH 5.6 | 54mV | pH 5.0-12 | Na+ 10.4, K+ 9.8, Ca++ 11.1 |
| TIPK |
Potassium | Yellow | 0.1M KCl | 54mV | 0.1 - 10-4.5M | Na+ 4.0, Ca++ 3.9, Mg++3.0 |
| TIPTPP |
TPP+ | Purple | 10mM TPP+ | 54mV | 0.001 - 10-4M | K+ 6.0 |
G.G. Kramarenko, S.G. Hummel, S.M. Martin, G. R. Buettner "Ascorbate Reacts with Singlet Oxygen to Produce Hydrogen Peroxide" Photochemistry and Photobiology 86. 2006: 1634-1637