This website uses cookies to ensure you get the best experience on our website.
Read more
Exploring the Intracellular World
April 24, 2013

Contributing Author: Dr. Steve Andre (Muscle Physiologist)
All living cells maintain a difference in electrical charge between the solutions that are inside and outside the cell membranes. The voltage difference across the cell membrane is usually at a steady level known as the resting membrane potential. The resting membrane potentialis produced by the differential distribution of ions on either side of the membrane. In muscle fibers, the potassium concentration inside the cell is over 50 times greater than the ion’s concentration in the extracellular fluid. On the other hand, the concentration of sodium is 10 times greater outside the membrane than inside. The concentration gradient of potassium is greater and in the opposite direction to the sodium concentration gradient. The ion concentrations are governed by three factors:
- Sodium-potassium pump
- Relative rates of diffusion of these ions down concentration gradients
- Attraction and repulsion of opposite and like charges
Sodium/Potassium Pump
The sodium-potassium pump actively moves sodiumout of the cell and potassium into the cell. The cell naturally has more K+ outside the cell and Na+inside the cell. The pump establishes and maintains the concentration gradients of these two ions across the cell membrane. The concentration gradients form an ionic battery across the membrane, and the sodium-potassium pump keeps that battery charged. To view an animation of this process, see:
The resting membrane potential, which ranges between -50 to -90mV in most cells, results from potassium diffusing. Because of its large concentration gradient, potassium diffuses faster across the membrane than any other ion. When potassium diffuses out of a cell:
- Excess negative charges occur inside the cell
- Extracellular potassium ions attract negative ions to follow them
- Extracellular potassium ions push positive ions on the outside back into the cell.
Since these other ions have relatively low permeabilities, they don’tmove as rapidly as the potassium ions. Therefore, these other ions haveonly a small effect on the neutralization of the negative potential inside the cell. The net movement of the potassium is also limited by the potential difference that builds between the inside and the outside of the cell. The negativity inside the cell holds back the positive potassium ions. At equilibrium, a potassium ion is pushed out of the cell through a channel by a chemical force (concentration gradient) thathas the same magnitude as the electrical force (negative potential) pulling the ion back into the cell. The membrane potential depends upon the concentrations of the different ions across the membrane and the relative permeability of the membrane to these ions.
Glass Microelectrodes
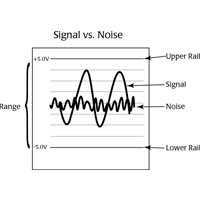
Resting membrane, action, slow receptor and synaptic potentials are measured with an intracellular electrometer like WPI's Duo773, using fine-tipped, fluid-filled glass micropipettes known as microelectrodes.
Microelectrode Amplifier
It is critical that the impedance of the cell, the microelectrode andthe amplifier be properly matched. Otherwise, the signal can be lost. To preserve the amplitude of the signal, a high impedance DC amplifier, known as an intracellular electrometer or a microelectrode amplifier, like WPI’s Duo773, is used in the circuit between the microelectrode and the recording unit.
This type of amplifier matches the impedance of the microelectrode tothe input impedance of the recording unit through a circuit known as a voltage follower. Electrometers usually have input impedances that are equal to or greater than 1010Ω. As a rule, the input impedance of an electrometer should be at least 100 times greater than the impedance of the microelectrode being used for recording. The small cross-sectional area of the microelectrode tip greatly reduces the flow of current through the electrode. So, it is said that this type of electrode has high impedance. This means that measuring potentials from smaller cells using microelectrodes with smaller tips and higher impedances requires the use of electrometers with higher input impedances.
In addition to having high input impedances, electrometers have low output impedances that more closely match the low input impedances of the recording units. This means that the actual amplitudes of the membrane potentials recorded using electrometers are the amplitudes displayed by recorders. Since these signals require little or no amplification, electrometers only need output gains of X1 or X10.
For normal resting action potentials (–50 to –90mV), use an X1 gain. For synapse potentials, which usually fall in the 5-10mV range, use an X10 gain.
NOTE: Electrometers do not pick up noise from electrical radiation as easily as other types of amplifiers.
A good quality intracellular electrometer has a few features, like a capacitor compensation circuit and a "tickler."
Capacitor Compensator
The capacitor compensation circuit, also known as the bridge, neutralizes the effect of the cell membrane, which usually acts as a capacitor by holding a charge. When an electrical pulse is injected intoa cell, the injected square waves have rounded corners because the cellmembrane dampens the injected current. Using a capacitor compensation circuit sharpens the corners of the injected square wave by depolarizingthe cell membrane. The end result of using a capacitor compensation circuit is a more effective injected pulse. The injected action potentials result in quicker response time and a recorded wave that is true to the one injected. The capacitor compensation circuit subtracts out the effect of the cell membrane on the injected potential.
Tickler
The tickler sends a quick, sharp pulse of current through the microelectrode. Also known a ringer, the tickler has two purposes. If the microelectrode is outside a cell, the tickler can be used to clean the tip of the electrode. When the microelectrode is pressed up against the cell so that a small dimple is formed in the cell, invoking the tickler helps the microelectrode penetrate the cell membrane.

Close